MOJ
eISSN: 2576-4519


Review Article Volume 1 Issue 3
Faculty of Biofarming, John Naisbitt University Belgrade, Serbia
Correspondence: Dragomir Lukac, Faculty of Biofarming, John Naisbitt University Belgrade, Serbia
Received: October 23, 2017 | Published: October 25, 2017
Citation: Luka? D, Mi?evi? B, Könyves T. Transgenes application and its barriers in contemporary animal husbandry. MOJ App Bio Biomech. 2017;1(3):104–108. DOI: 10.15406/mojabb.2017.01.00016
Efficiency of the genetic engineering application in contemporary livestock production is very low, actually only 1%. The main reason for failure lies in the fact that so far, little attention was paid to gene position effects, as well as fidelity of the numerous DNA polymerases active in eukaryotic cells. Any change in the DNA sequences, through insertions, deletions or nucleotide mutation, affects stability of the genome. This is particularly felt in DNA replication, where the information’s transfer to the off springs calls into the question. It is necessary to find new approach to overcome these barriers. Is there are unique way to solve those two barriers. In recent years in the use are Sleeping Beauty transposase, Zn-finger nuclease, lent viral integrase, site-specific recombinase, etc. Perhaps the solution lies in introducing the transgenes into predetermined genome loci via site specific phage фC31 integrase into pronuclear of the target animal. In the context of transgenes is better to talk about the bio DNA, corresponding to host DNA, and labDNA, DNA artificially constructed in laboratory.
Keywords: gene position effect, genome integrity, transposase, integrase, zn-finger nuclease, recombinase
Two sides are present in different modification of genome: foreign DNA and host genome. In this context, is better to talk about the bioDNA, which corresponds to the naturally evolved deoxyribonucleic acid (host DNA), and labDNA, which corresponds to the artificially DNA constructed in the laboratory. What can be done, and which constructs are allowed that lab DNA survived and continued in the next generations? Foreign DNA (labDNA), like any other DNA, holds a vital energy that should be incorporated into a genome, otherwise is doomed. It seeks a place and a way to incorporate in order to replicate, transfer their material to their offspring, no matter what the stranger is in the domestic system. It will be no surprise if the success of genetic engineering is, among other things, depended on the “adaptive evolution” of the labDNA. The capacity is enormous the best examples are network-like mode of RNA virus evolution, i.e. adaptation to new conditions. There are novel virus genome develop by recombination between unrelated groups of RNA and DNA viruses.1 Homologous recombination could be used to specifically modify genes in mammalian cells.2,3 Diemer GS,1 was discovered that chromosomally normal cell cultures could be established directly from early mouse embryos. These cells are now referred as embryonic stem (ES) cells.
All genes present in any genome may be accessible to modification by homologous recombination. Genes could be targeted in cultured cells and the targeted cells are, in most cases, embryonic stem cells (ESC). These two facts should be connected together, with one hand genetic homologous recombination, on the other hand ES cells. All the pieces were at hand to begin generating gene targeted embryonic stem cells. For insertion of foreign DNA, gene position effect and genome integrity are barrier for proper expression efficiency of interested transgenes. Predominant methods used to produce lab-animals have several limitations: genome integrity, insertion site, and copy number of the transgenes cannot be controlled. Single-copy transgenes is can be expect with retroviruses, and transposons, but the transgenes is integrated throughout the genome. One of the best methods in use is site-directed recombinase, which can span both of these obstacles.
Gene position effect
Gene position effect and faithful preservation of genome integrity is the huge obstacle to the gene targeting success. Numerous attempts have been applied to overcome these biological barriers. In eukaryotes a considerable proportion of the genome is represented by heterochromatin. The gene position effect is reflected in gene rearrangements, translocations, as a result of such changes gene may be integrated into chromosomes active zones (euchromatin) to the inactive zones (heterochromatin) and become inactive. There are constitutive and facultative heterochromatins. Constitutive heterochromatin is predominantly positioned in pericentromeric and telomeric regions which is rich in repetitive sequences predominantly consisting in transposable elements. Facultative heterochromatin represents transiently condensed and silenced euchromatin. One of the examples is the inactivated X chromosome in female mammals. The gene position effect may cause disruption in the activity of several genes close to heterochromatin, the influence of heterochromatin is always in the direction of the nearest euchromatin gene. This means that it is very important to choose the phase of synchronized cells division for insertion of the gene of interest. Gene is not necessarily silenced by the effect of heterochromatin, because the heterochromatin did not spread across this gene early in development, when heterochromatin firs formed. It means that state of transcriptional activity of gene is inherited, once determined by its chromatin packaging in the early embryo.4,5 Genes can be integrated/transferred from the chromosome active zones to the inactive zone and become silenced, and vice versa. The reversibility of the position effect demonstrates that a given genetic change is due to the position effect rather than to genetic mutation. The heterochromatin is activated upon being transferred to the euchromatin and becomes cytological indistinguishable from the latter. Gene position effect describes also the variation of expression pattern exhibited by identical transgenes inserted in different sequences of DNA. The difference in expression is due to the neighboring enhancers. Each transgenic organism has the potential for a unique expression pattern, since each transgenes has a different location in the genome. In mammals, the insertion of the transgenes can trigger transcriptional silencing of the transgenes in order to protect the structure of host chromosomes.
Posibility to overcome GPE
Transpososase: Discussion must illustrate and interpret the review study.DNA transposons are naturally occurring mobile genetic elements that “copy and paste”–class I and “cut and paste” – class II, themselves to move from one genomic location to another unique site within the host genome. Movement of DNA segments resulting in rearrangement of genomic DNA, initiates when transposase forms a diametric DNA-protein synaptic complex with transposons DNA end sequences. A transposons-encoded transposase recognizes the inverted terminal repeats flanking a transposons and catalyses the transposition of the element into the genome. Transposons are found in many major branches of life. They may have originated in the last universal common ancestor, or arisen independently. While some transposons may confer benefits on their hosts, most are regarded as selfish DNA parasites. Cell defends against the proliferation of transposons by piRNAs (piwot-interacting), siRNAs (small interfering)6 which silence transposons after they have been transcribed. Chicken primordial germ cells, for example, resist deliberate genetic modification, likely by silencing the introduced genes in the genome. Selection for transgenes integration into chicken primordial germ cells (PGC) genome and sequencing of the insertion sites revealed that the transgenes preferentially inserted into active promoter regions, implying that silencing prohibited recovery of insertion in other regions. This is one of the interesting way to cell to liberate from the transgenes.
Despite the evidence for transcriptional silencing in PGCs, gene targeting of a no expressed gene was also achieved. Genetically modified chickens serve as models for studying developmental biology, as bioreactors for therapeutic products, as a model for disease resistance to enhance agricultural production. Results form study of7 shown that PGCs can be manipulated efficiently using transposons vectors. They used piggyback and Tol2 transposons to modify PGCs stably. Tol2 transposons was five time more efficient than the piggyback transposons in modifying chicken PGCs.7 was shown that, contrary to the others, insulator DNA, sequences that shield regions of DNA form epigenetic silencing, were not required in the integrated transposons for transgenes expression. PGCs containing integrated transgenes were able to colonize the gonad of host embryos and form functional gametes that produce transgenic offspring. This transgenic chicken should become important contributors to health, science, and agriculture.
The article of8 addresses the question about the behavior of transgenic animals in the wild population. No autonomous transposons insertions can be remobilized by exposure to a wild population’s transposase, when transgenic insects are release in environment. A method was developed to stabilize transposons insertion through post-integration excision of one end of the transposons. For this purpose, they used piggyback transposase which does not necessarily use available pair of suitable terminal sequences. To generate transposons-free insertion, composite element with central domain flanked by two short no autonomous piggyback elements are used. The resulting insertions lack transposons sequences and are therefore impervious to transposase activity.
Sleeping beauty transposase: Discussion must illustrate and interpret the review study. Sleeping Beauty (SB) transposons system is synthetic DNA transposon that was constructed to introduce precisely defined DNA sequences into the animal genome. SB transposase inserts transposons into TA dinucleotide base pair in a host genome. In the process of integration TA site is duplicated, and this duplication is a hallmark of transposition. All of the transposons identified in the mammalian genomes are non-autonomous because the transposase gene is non-functional and unable to generate an enzyme that can mobilize the transposons. This means that the host cell possess the mechanism that regulates activity of transposase. The reconstruction of SB transposase was based on the concept that there was a primordial transposase genes found in fish that have been inactive more than 10 million years due to the accumulated mutations. A putative ancestral consensus sequence was predicted, and over the decade SB construct was increased which contains all of the motifs required for function.9 SB transposons can be use to carry a transgenes and associated elements that confer transcription regulation for expression at a desired level in specific tissue. SB can be used to discover the new gene function, to delivering DNA sequences this way, that gene is “knocked out”. SB transposons combine the advantages of viruses and plasmids. The use of non-viral vectors avoids some of the defenses that cells evolved against vectors. There are good few problems with most methods for delivering DNA to the genome using plasmids. Uptake of plasmid into cells is difficult, expression of transgenes from plasmid is brief due to cellular response that influence expression, it should be avoid multiple integrations which results in switch off expression of transgenes, using plasmids is much less efficient than using viruses. Using SB can provide useful levels of success of expressing transgenes for entire animals. The long-term stability of labDNA insertion can be tested when insects, for example, are released in the environment. Population in wild might contamine the laboratory organisms with exogenous transposase insertion and remobilized transposons. was stabilized transposons insertion through post-integration excision of all transposons sequences from the lab DNA, rendering it as inert to transposase as any other bioDNA. Hoping that such a approach may permit genetically modified insects to resist to natural selection.
Lent viral integrase: In recent years lent viral vector application have received a great attention including gene therapy, generation of lab-animals, and the stable dalivery of RNA interference molecules. The main reasons for this are the qualities that lent virus possesses efficiently transduction of nondividing cells, ignoring the role of replication, - shuttle large genetic cargo, long labDNA, - maintain stable long-term labDNA expression. A retroviral vestor system based on the HI-viruses was developed that could mediate stable in vivo gene transfer into many cells types. So far brain, liver, muscle, hematopoetic stem cells, terminally differentiated neurons, have been successfully transduced with lentiviral vectors carrying a variety of genes.10,11 The HIV-1 proteins matrix, Vrp, inegrase are responsible for viral genome import in non-dividing cells. But, a sequence within pol gene, containing structural elements associated with the progress of reverse transcription, is also required for gene transfer by lentiviruses.12 Once you have all this cargo loading into cell it is only matter of time when these lentiviruses will turn into viral diseases. Even when Follenzi state: Full rescue of this step in lentivirus-based vectors improves performance for gene-therapy application“. We should not lose sight of the resourcefulness of the viruses.1 When retroviruses integrate in the bio DNA they can cause insertion mutagenesis. The consequences depends entirely on the location within the bioDNA where viral genome is inserted; either within the gene, promoter region, repressor gene, enhancer; leading to altered cellular activity. This integration can be avoided using defective viruses, leaving their genome like episomes, free in the nucleus.
A key challenge for labDNA based on retroviral and lent viral vectors is to minimize insertion mutagenesis. In vitrostudies have shown that integration-deficient lent viral vectors can mediate stable transduction. Integrase bounds to the attachment (att) region of the LTR to catalyze the covalent linkage with cellular DNA. Nearly half of the proviral DNA becomes episomal; the two major circular episomal forms results from no homologous end-joining and homologous replication, this is a potential for use of non integrated lent viruses. The hot spots where is applicably to modified LV can be seen in viral poll gene (integrase), and LTR regions. Viral cis-acting DNA elements, central polyp urine tract sequence (cppt) and the woodchuck post regulatory element (WPRE), are included in efficiency of transduction. The cppt is a small DNA fragment in the poll gene usually cloned 5’ to the internal promoter region, whereas the WPRE is cloned 3’ to the inserted labDNA so that is in close proximity to the poly(A) stretch in the 3’-LTR.13 Another important LV transfer plasmid is a 400 bp deletion in the U3 region of the 3’-LTR, which deliberates 5’-LTR RNA poll II promoter activity following integration.14,15 From the other side, there are LTR sequences as 5’-LTR which acts like RNA poll II promoter, 3’-LTR acts to terminate transcription and promote polyadenilation, and the LTR sequence that recognizes sequence in bio DNA is necessary for integration.
Changes in LTR or in pol gene by introducing combinations of mutations may disable integrase protein itself or alter the integrase recognition sequence (att) in the viral LTR. To overcome risk of insertion mutagenesis it is possible by developing a non integrative LV vectors. Philippe et al.16 are constructed LV vector with defective integrase by replacement of the 262 RRK motifs by AAH. This derivative vector drives efficient labDNA expression in dividing and non dividing cells in vitro. They have estimate that the mutant vectors integrated 500-1250 times less frequently than wild type vectors, and it retains in episomal states. In that way LV vectors has great potential to overcome insertion mutagenesis, and be applicable in efficient labDNA transfer in bioDNA. One of the interesting lent viral vectors is simian immunodeficiency virus-based vectors. Nonhuman primates are appropriate for the study cognitive functions and brain disorders. But, human disease do not occur naturally in monkeys, therefore the transgenic animals are needed. In their experiment Yuyu et al.16 Produced four infant rhesus monkeys from four singleton pregnancies, of which two expressed EGFP (widely used) throughout the whole body. This is a very encouraging sign for the future use of lab-animals for gene therapy.
Genome integrity: Depending on the type of damage inflicted on the DNA's double helical structure, a variety of repair strategies have evolved to restore lost information. If possible, cells use the unmodified complementary strand of the DNA or the sister chromatid as a template to recover the original information. Without access to a template, cells use an error-prone recovery mechanism known as translation synthesis as a last resort. There are 5 known prokaryotic family and 15 known eukaryotic types of DNA polymerases with different endo-, and exonuclease activity, participating in fidelity of DNA replication.
Damage to DNA alters the spatial configuration of the helix, and such alterations can be detected by the cell. Once damage is localized, specific DNA repair molecules bind at or near the site of damage, inducing other molecules to bind and form a complex that enables the actual repair to take place.
ncRNA and genome integrity: Some non-coding (nc) RNA are processed by DICER and DROSHA R nose to give small double-stranded RNAs. Upon exogenous DNA influence, DNA-damage response (DDR) is activated at a single inducible DNA double-strand break (dsb). To repair this type of damage, DICER and DROSHA – dependent small RNAs (DDRNAs) are acting at genomic location of DNA break. Without DDRNAs cell is not alerted to DNA breaks and there are not respond to repair damage. Almost the entire genome is transcribed into RNA whose transcriptome is comprised of many low expressed non-coding RNA. All of these low expressed short RNA (20-25 nucleotides), contribute to regulate the functional organization and expression of the genome, and like in the case of DDRNAs integrity of the genome.
To the monitoring of DNA, a new dimension is given by discovering short non-coding RNA (ncRNA) molecules that ensure the stability of the genome.17 So in addition to the water and ncRNA contribute to the integrity of the genome. There are several classes of small RNA; micro RNA (miRNA), conventional small-interfering RNA (siRNA), and single, stranded RNA (ssRNA). Regarding the genome integrity, two of them are of great importance, siRNA and ssRNA. siRNA of ~21 nucleotides are produced through defence against external nucleic acids. ssRNA are processed to ~27 nucleotides Piwi-interacting RNA (piRNA). It is probably that piRNA function as master controllers of transposable elements (transposons).
Possibility to overcome genome integrity
Zn-finger nuclease: ZFN is another arsenal that labDNA used to integrate in the bioDNA. ZFN are one of the most powerful and painless ways to change the structure of DNA without big loads on the integrity of the genome and positional effects of genes. ZFNs are artificial restriction enzymes, chimeras of a DNA-specific binding domain (Cys2 His2 zinc-finger protein) and DNA-cleavage endonuclease Fok I.18 The principle is that ZFNs induce site-specific double-strand break (dsb) in bioDNA that can be repaired by error-prone nonhomologous end joining (NHEJ) or by error-free homologous recombination.19 Introducing a dsb in eukaryotic genome stimulates DNA repair mechanisms. NHEJ can produce deletion or insertion of short sequences at the break.20 Fok I restriction end nuclease from Flavobacterium okeanokoites consisting of N-terminal DNA-binding domain (5’-GGATG-3’ : 3’-CATCC-5’), and C-terminal cleavage domain which cleaves the first strand 9 nucleotides downstream and the second strand 13 nucleotides upstream of the nearest nucleotide of recognition site.21,22 Each finger bind firstly 3 bp, the component sites are 9 bp in length and the optimum for paired sites is an inverted orientation with a spacer of 6 bp.23 If all nucleotides in the mutated target are contacted specifically, these live 18 bp recognition sequence, long enough to be unique even in a complex genome. If ZFNs finds the recognition sequences, and if separation between the component 9-mers is not a 6 bp, than corresponding linker between the binding and cleavage domains should be added. ZFN approaches greatly facilitated the ability to direct mutations arbitrary to mutated sequences without the need to alter bioDNA in advance.
Site-specific recombinase
Since the initial discovery that recombinase can be used in genomic engineering,24 the recombinase-mediated cassette exchange, one of the technology in the field of reverse genetics, is of increasing relevance.25 To effectively resolve complex labDNA insertion, and to avoid epigenetic influence, site-specific recombination technology enters the field. Site-specific recombinase are grouped into two families: the tyrosine recombinase (such as Cre, Flp), and serine recombinase (Tn3 resolvase, фC31, Bxb1, R4 integrase). One of the best examples is the фC31 integrase in mice.26 In their experiment, фC31 integrase (Figure 1) were used to catalyze recombination between one or two attB sites in a labDNA with one or more tandem attP sites that they previously inserted into specific loci in mice bioDNA. Via pronuclear injection Tasić et al.27 received single-copy insert into predetermined chromosomal loci with high efficiency (up to 40%).
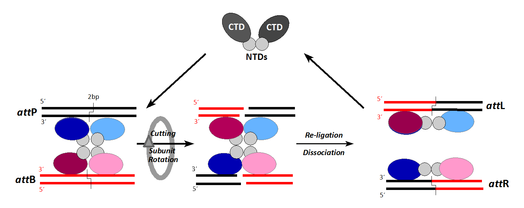
After many years of wandering in search of the best ways to make transgenic animals, perhaps we are on a track to achieve. Latest developments in molecular biology have made it possible to apply new techniques in scoring lab DNA, without major changes in the bioDNA. To overcome gene position effect, genome integrity, and copy number of the transgenes, in the application are the latest technologies. For targeting the gene of interest, perhaps different recombinase can be primarily used (Cre and Flp). To avoid any impact on the integrity of the genome, ZFNs and фC31 recombinase-integrase can be used in the future.
None.
Author declares that there is no conflict of interest.

©2017 Luka?. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.