MOJ
eISSN: 2576-4519


Research Article Volume 2 Issue 2
Department of Mechanical and Aerospace Engineering, University of Uyo, Nigeria
Correspondence: Paul Aondona, Department of Mechanical and Aerospace Engineering, University of Uyo, Uyo, PMB 1017 Uyo, Akwa Ibom State, Nigeria
Received: March 30, 2018 | Published: April 9, 2018
Citation: Ihom, Aondona P. The efficacy of carburizing compounds with different carbon source and added industrial energizers for surface treatment of mild steel for mechanical property improvement. MOJ App Bio Biomech. 2018;2(2):130–135. DOI: 10.15406/mojabb.2018.02.00055
The Efficacy of Carburizing Compounds with Different Carbon Source and added Industrial Energizers for Surface Treatment of Mild Steel for Mechanical Property Improvement’’ has been studied. The research work involved material sourcing and carburizing compounds preparation. Five compounds were prepared in carburizing steel boxes in which mild steel specimens were embedded covered and sealed with clay. Electric muffle furnace was used for the carburization process. The pack carburization boxes were inserted in the furnace at operational temperature of 920ºC and holding time of 8 hours. They were then removed and the specimens quenched in water. The specimens which were earlier on prepared by machining using lathe were cut into two and the cut-side was ground, polished and etched for metallographic analysis. For hardness test; the carburized specimens were not etched. The result of the work showed that carbon source determines the efficacy of the carbon material as a carburizing compound in pack carburizing. It was also noticed that charcoal alone produced case depth of 0.08mm and surface hardness of 58.42HRC, while coal had case depth of 0.06mm and surface hardness of 54.42 HRC under the same conditions of holding time and carburizing temperature. The addition of industrial chemical energizers drastically improved the efficacy of the carburizing compounds, but the combination of coal and charcoal to energizers did not lead to any synergistic effect in terms of high case depth and surface hardness. The best carburizing compounds were compounds C and D because of the presence of the energizers; they had surface hardness of 64.44 HRC and 64.39 HRC respectively. In the absence of energizers; the charcoal performed better than the coal.
Keywords: carbon source, energizers, efficacy, surface treatment, mild steel, carburizing compound
The fixed carbon in different carbon sources in most cases is not the same. The carbon concentration in wood charcoal, is not the same with coa, l and neither is it the same with bone charcoal or sugar cane charcoal. Carbon availability at the surface of mild steel which at high temperature normally provide nascent carbon during pack carburization determines the success of carbon diffusion into the steel surface at austenite temperature. Carbon source with higher fixed carbon will definitely make more carbon available at the steel surface than sources with low amount of carbon. Energizers raise the carbon potential of the carburizing pack making a difference in the efficacy of the process; leading to higher case depth than when only carbon source is used1‒5 It might be interesting to find out how different carbon sources might influence casehardening with or without the presence of industrial energizers in the carburizing compounds.
According to Ihom et al.,6 the use of surface treatment to improve corrosion resistance, wear resistance, heat resistance, ornamental properties, functional properties, friction reduction, hardness improvement etc is now a known practice. Surface treatment, may be applied at the completion (or finishing) of mechanical fabrication of components and are in every sense finishing processes. Advances in surface finishing technology have given rise to high performance machines and automobiles. Ihom et al.7 cited that ‘’dead mild steels containing up to 0.15% C are used for general presswork and other applications where high ductility is necessary in forming. Mild steel containing 0.15-0.3% C are referred to as wrought. Wrought forms are used as rolled stocks (RSJ) and other structural members, shafting, levers, and various forgings. Steels used for sand castings usually contain 0.3-0.35% C they are also considered as mild steels.1,3 These set of steels described above do not respond to hardening heat treatment, but only annealing treatment for grain refinement Saita.8 However, given their wide area of application they do encounter service limitations, one of such limitations have to do with low hardness and wear resistance particularly when used as shafts and other rotating parts of machines. To improve on the hardness and wear resistance, several casehardening methods are normally used which include nitriding, carbonitriding, cyaniding, carburizing etc.. just to mention a few surface hardening techniques. These technique are used to improve the surface hardness of mild steel. The technique involves the use of various raw materials for the impregnation of different elements in the surface of the mild steel to improve the hardness and wear resistant properties. This normally improves the service life of the part made of mild steel Aigbodion & Hassan,9 and Ihom et al.7 In this particular research work the efficacy of carburizing compounds with different carbon sources and industrial chemical energizers for surface treatment of mild steel is being investigated. This forms the core objective of the research work to establish the efficacy of carburizing compounds with different carbon source and industrial chemical energizers for surface treatment of mild steel. It will be interesting to know to what extent the fixed carbon of the carbon source influence the efficacy of the carburizing compound.
Materials
Materials used for the research included; acetone, clay, nital solution, emery cloth, mild steel rod of 20 mm diameter, coal, charcoal, BaCO3, Na2CO3,NaOH, water, and polishing powder. All were sourced locally within Nigeria. The mild steel was obtained at Delta Steel Company, Aladja. The chemical composition is shown in Table 1 and (Figure 1) (Figure 2) show charcoal and coal that was used in the work. The equipment used were hacksaw, lathe machine, Vernier calipers, grinding and polishing equipment, electric furnace, heat resistant steel boxes, hardness testing machine (Rockwell), and metallurgical microscope.
Method
C |
Si |
Mn |
P |
S |
Cr |
Mo |
Ni |
Sn |
Cu |
V |
0.13 |
0.15 |
0.47 |
0.043 |
0.006 |
0.01 |
0.01 |
0.01 |
0.001 |
0.03 |
0.002 |
Table 1 Chemical Composition of Mild Steel from Delta steel Company
Compound |
Composition |
A |
100% Coal |
B |
100% Charcoal |
C |
55% Coal, 15% CaCO3, 10% Na2CO3, 20% BaCO3 |
D |
55% Charcoal, 15% CaCO3, 10% Na2CO3, 20% BaCO3 |
E |
25% Coal, 30% Charcoal, 15% CaCO3, 10% Na2CO3, 20%BaCO3 |
Table 2 Different Carbon Sources with Industrial Chemical Energizers in some Compounds
Results (Figures 4-11)
Microstructural analysis (12,13)
Discussion
Figure 4 is the hardness profile of the un-carburized specimen against distance from the surface of the specimen. The plot is almost a straight line showing the uniformity and homogeneity of the un-carburized mild steel microstructure. The little variations may be from scales or inclusions. This agrees with previous works by several authors who made similar observations Ihom,10 Ihom et al.,7 Asuquo & Ihom,11 Ihom et al.12 Figure 5 is the hardness profile of carburized mild steel using compound A against distance from the surface of the specimen. Compound A, is only coal. It produced a case depth of 0.06mm. Figure 6 is the hardness profile of carburized mild steel using compound B against distance from the surface of the specimen. The plot produced a case depth of 0.08mm. Compound B is just charcoal. Figure 7 is the hardness profile of carburized mild steel using compound C against distance from the surface of the specimen. The plot produced a case depth of 0.09 mm. Compound C was made up of 55% Coal, 15% CaCO3, 10% Na2CO3 and 20%BaCO3. Figure 8 is the hardness profile of carburized mild steel using compound D against distance from the surface of the specimen. The plot produced a case depth of 0.11mm. The compound D was made up of 55% Charcoal, 15% CaCO3, 10% Na2CO3 and 20% BaCO3. Figure 9 is the hardness profile of carburized mild steel using compound E against distance from the surface of the carburized specimen. The plot produced a case depth of 0.08 mm. The carburizing compound E is made up of 25% Coal, 30% Charcoal, 15% CaCO3, 10% Na2CO3 and 20% BaCO3. Figure 10 is the bar chart of case depth variation with the various carburizing compounds used in the research work. The chart shows that the highest case depth of 0.11mm was produced by compound D followed by the case depth value of 0.09mm produced by compound C. Compounds B and E had the same case depth value of 0.08mm. Figure 11 is the bar chart of surface hardness variation of the various carburizing compounds used in the research work. Carburizing compound C had the highest surface hardness of 64.44HRC, followed closely by carburizing compound D with surface hardness value of 64.39 HRC. Compound E followed with surface hardness of 59.42HRC which was also closely followed by compound B with 58.42 HRC. Carburizing compound A came last with surface hardness value of 54.42HRC. Figures 4‒11 clearly presents the performance of the carburizing compounds as case hardening materials. Their efficacy has been shown from the case depth and surface hardness they produced on the mild steel specimens.
Under the same conditions of holding temperature of 920 ºC and 8 hours holding time; coal alone produced a case depth of 0.06mm and surface hardness of 54.42HRC. Charcoal alone produced case depth of 0.08mm and surface hardness value of 58.42 HRC. In compounds C and D industrial chemical energizers were added individually to coal and charcoal. Compound C which had coal gave a case depth of 0.09 mm and surface hardness of 64.44 HRC and Compound D which had charcoal produced a case depth of 0.11 mm and a surface hardness of 64.39HRC. Here again the compound with charcoal had a better case depth and a competitive surface hardness with the compound containing coal. In compound E the two sources of carbon were combined with industrial chemical energizers to verify their synergistic effect. Unfortunately, the compound E produced the same case depth of 0.08mm just like charcoal alone produced. The surface hardness was 59.42 HRC which was still not so different from compound B surface hardness value of 58.42HRC. There was therefore no synergy in combining the two sources of carbon. However, it was observed that in all the cases where the energizers were added the efficacy of the carburizing material was enhanced. The experiment was conducted under the same conditions of time and temperature, so for one carbon source to be better than the other, it has to do with the carbon content of the source and its carbon potential. This position has been upheld by several researchers who have done similar works Ihom et al.13 (Figure 12) (Figure 13) shows the microstructure of un-carburized mild steel and carburized mild steel that has been carburized using the carburizing compounds in the work. The two plates are just explaining why Figure 4 is different from Figures 5‒9 The un-carburized mild steel has a homogeneous microstructure, whereas the carburized mild steel has a hard carbon- rich dark surface and a soft-light carbon impoverished core Azoro.5
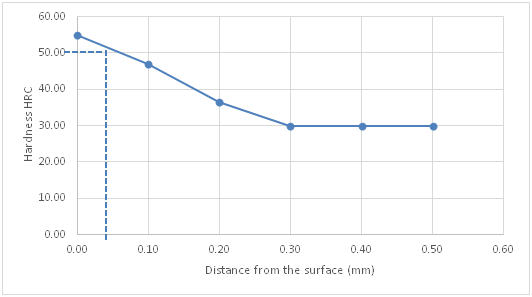
Figure 5 Hardness profile of carburized specimen using compound a against distance from the surface.
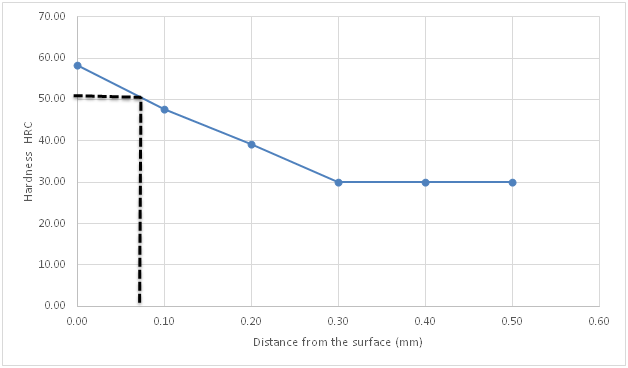
Figure 6 Hardness profile of carburized specimen using compound b against distance from the surface.
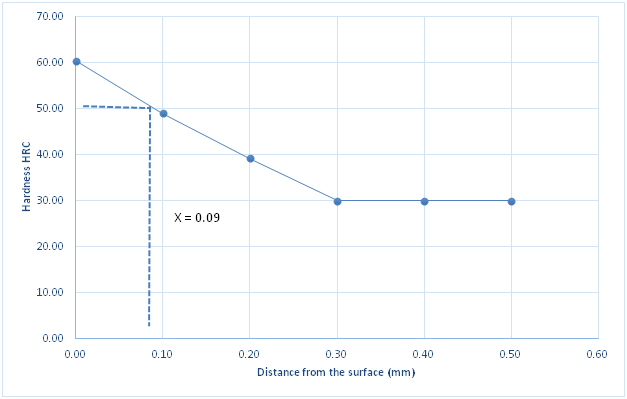
Figure 7 Hardness profile of carburized specimen using compound c against distance from the surface.
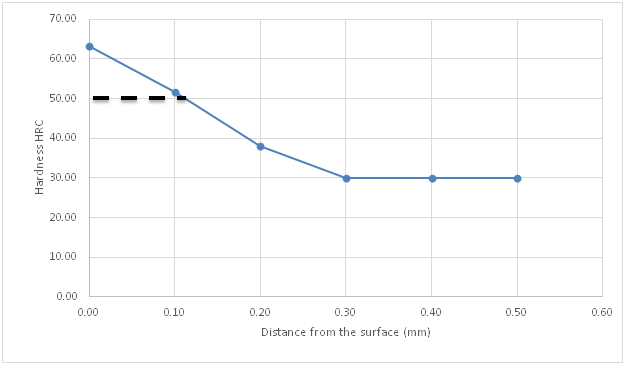
Figure 8 Hardness profile of carburized specimen using compound d against distance from the surface.
The work ‘The Efficacy of Carburizing Compounds with Different Carbon Source and Industrial Chemical Energizers for Surface Treatment of Mild Steel’’ has been undertaken. The following findings and conclusions have been drawn from the work:
The author of this research work wish to acknowledge the Technologists in the Faculty of Engineering workshop, the Technologists in the Civil Engineering Laboratory, and Agricultural Engineering Laboratory; all of the University of Uyo. I also want to thank the Technologists in Federal University of Technology, Owerri and Michael Okpara University of Agriculture, Umudike where the hardness tests and microstructural analysis were done. I appreciate all of you for your time and assistance.
The authors declare that there is no conflict of interest.

©2018 Ihom,. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.