MOJ
eISSN: 2576-4519


Research Article Volume 7 Issue 1
1Director of the PROBIOTEC-FORAPIS Research Group, Full-time Professor, Animal Science Program, Faculty of Animal Sciences, University of Nariño, Colombia
2Researcher of the PROBIOTEC-FORAPIS Research Group, Animal Science Program, Faculty of Animal Sciences, University of Nariño, Colombia
Correspondence: Henry Jurado-Gámez, Zoot, Esp, M.Sc, Ph.D, Director Grupo de Investigación PROBIOTEC-FORAPIS, Profesor Titular de Tiempo Completo, Programa de Zootecnia, Facultad de Ciencias Pecuarias, Universidad de Nariño, Pasto, Colombia, Tel +573128333159
Received: October 31, 2023 | Published: November 14, 2023
Citation: Jurado-Gámez H, Solarte APD, Argoti ICF. Microencapsulated Lactobacillus lactis against Salmonella typhimurium, its viability under simulated gastrointestinal conditions and cell adhesion. MOJ App Bio Biomech. 2023;7(1):205-209. DOI: 10.15406/mojabb.2023.07.00196
There is significant evidence of the positive health impacts of probiotics in humans and animals, which is why their application has potentially increased in recent years, especially in the food and pharmaceutical industry. In this context, their inhibitory potential has also been explored as a strategy to ensure food safety and biocontrol of pathogens relevant to public health. The objective of the research was to determine the inhibitory effect of microencapsulated Lactobacillus lactis on Salmonella typhimurium and its viability under simulated gastrointestinal conditions. To characterize the lactic strain, a preliminary experimentation was carried out, and based on this information, four inhibition methods were proposed, with different concentrations of the probiotic bacterial supernatant, then the lactic strain was subjected to simulated gastrointestinal conditions, with different pH levels and % of bile and bile salts, and finally the in vitro adhesion assay was performed. Inhibition halos greater than 2 mm (PADS) were found, optimal viability values were observed (3.0x108UFC/mL), adhesion capacity of the strain in cell cultures was evidenced.
Keywords: microencapsulatión, inulin, viability, inhibition, health, probiotic
MB, Mitochondrial diseases; mtDNA, mitochondrial DNA; MRI, magnetic resonance imaging; GTCS, generalized tonic-clonic seizure; HSV, herpes simplex virus; ENMG, electroneuromyography
The regular intake of microorganisms can provide multiple benefits, such as the maintenance of health and the prevention of various pathologies. In this regard, Gómez-López1 mentions that, given the growing boom of these products in the market and their industrial and commercial potential, it is an ethical, scientific and technological imperative, to study their efficacy, bioequivalence, safety and routes of administration, and, in general, to guarantee their clinical use with the highest safety margin for patients. Likewise, it is necessary to establish the standards and controls of the production processes that allow guaranteeing the high quality of the product. The same author also mentions that in Colombia, the Ministry of Health and Social Protection and the National Institute for Drug and Food Surveillance (Invima) -institutions in charge of establishing the regulatory framework for these products- have regulations for the evaluation of drugs with probiotics, prebiotics or synbiotics and also on the requirements for their production and storage. As a result, several products considered as probiotics or prebiotics, and dietary supplements with probiotics, synbiotics or both, have been approved for more than 10 years.
In this order of ideas it is imperative in the food industry to find strategies that allow the efficient use of beneficial microorganisms, in this way, one of the techniques widely recommended for the conservation and use of lactic acid strains in spray drying, according to what has been reported in different investigations this technique has been studied for several decades and has been in constant innovation, becoming one of the most important trends for the food industry,2 cosmetics,3 pharmaceuticals,4 textiles,5 among others. Shahidi and Qing Han,6 in this regard mention that spray drying is one of the oldest encapsulation techniques, with a beginning dating back to the 1930s, where the first applications focused on the microencapsulation of flavors, using acacia gum as a wall material or encapsulant. The spray drying microencapsulation technique is characterized by its low costs compared to other drying methods used,7 hence there is a wide diversity of applications, among which are the encapsulation of essences,8 powdered beverages,9 flavors,10 probiotics,11 dyes,12 among others.
Ríos-Aguirre and Gil-Garzón,13 state that due to its wide diversity of applications, microencapsulation will act as a protection mechanism, according to the chemical composition, polarity, solubility, physical and functional properties of each matrix, improving aspects such as:
The research took place in the laboratory of the PROBIOTEC-FORAPIS research group, located in the facilities of the Universidad de Nariño, Pasto, Colombia. The commercial lactic strain Lactobacillus lactis ATCC® 19435 and the commercial pathogenic strain Salmonella typhimurium ATCC® 29934 (MDM Scientific S.A, Medellín, Colombia) were proposed.
For the development of the research, a preliminary experimentation was carried out in order to characterize and know the kinetic behavior of the lactic strain, for which the inoculum adjustment procedure was performed at the maximum scale of MacFarland and incubation was carried out for 24 hours at a temperature of 37°C under aerobic conditions. During the kinetics, samples were taken every 4 hours in order to evaluate over time the kinetic variables: probiotic viability (Ln CFU/mL), sugar consumption (mg/L),14 protein production (mg/L),15 pH, and lactic acid production.
Additionally, assays for the evaluation of L. lactis growth at two temperature levels (35°C and 43°C) were performed according to Cai et al.,16 gas production assays, where the lactic strain was grown in MRS broth + anhydrous glucose (5%) in test tubes with inverted Durham tubes for 24 hours at 37°C. Finally, the catalase assay was carried out to determine the absence or presence of catalase, for which biomass of the lactic strain was taken on a slide and a drop of 30% hydrogen peroxide was added. When the enzyme was present, effervescence was observed and in the absence of the enzyme, no effervescence was observed.
Subsequently, the microencapsulation process of the lactic strain was carried out under the following operating parameters of the equipment: inlet temperature 145°C, outlet temperature 65°C, feed flow of 6 mL x sec, in a cycle of 1 hour and 30 minutes, thus, a suspension (500mL) was prepared consisting of the mixture of bacterial inoculum + inulin (15% p/p-37.5 g) + Maltodextrin (15% p/p-37.5 g). The probiotic powder was kept in metalized zipper bags at room temperature (18°C) for 40 days.
To determine the stability of the probiotic powder under storage conditions, we followed what was established by Rodriguez et al.,17 for such purposes, the determination of physicochemical and viability parameters were performed: viability (%), Aw, humidity (%), solubility (%), wettability (min) and microencapsulation efficiency (%), morphology and capsule size.
Within this context, the probiotic powder was subjected to tests where its viability under simulated gastrointestinal conditions and its adhesion capacity were evaluated. In this way according to the methodology proposed by Brodkorb et al.,18 and Guo et al.,19 1g of microencapsulated powder was taken and added to 100 mL of distilled water. (Phase I) 25mL of phosphate buffer solution (0.1 M; pH 6) and 10mL of 0.2 M HCl were added and shaken for 5 min. (Phase II) The pH was adjusted to 2 using 1M HCl, and 1mL of pepsin solution in 0.2 M HCl (concentration of 25 mg/mL) was added and mixed well. The vessel was sealed and incubated at 40°C for 120 min. (Phase III) 10 mL of a buffered phosphate solution (0.2 M; pH 6.8) and 5mL of a 0.6M NaOH solution were added to maintain a stable pH 6.8 (NaOH 1M), 1mL of a 100 mg/mL pancreatin solution (porcine, grade VI, Sigma n. P-1750), vessels were sealed and incubated at 37°C for 300 min. 1mL of sample was removed from each phase for plate counting.
For the determination of the adhesion capacity of the probiotic strain, slides with Sigma-Aldrich human mucin tissue (Mucin Tissue - Trol TM, AR-Med LTD- Runnymed Malthouse- TW20 9BD U.K) were used following the methodology proposed by Serna-Jiménez,20 where the results were classified according to the type of adhesion obtained: considered as negative adhesion, when there is no adhesion of bacteria, and will be considered as positive adhesion when adhesion of bacteria is observed.
Finally, to evaluate the inhibition capacity of the lactic strain against the pathogenic strain, 4 methods were used (i. impregnated agar disks, ii. impregnated PADS, iii. Disc diffusion, iv. Double disc diffusion with concentrations of 80 µL, 90 µL and 100 µL and 100 µL, respectively. The selection criterion as positive inhibition was greater than or equal to 2 mm halo, as established by Jurado-Gámez et al.21
The preliminary experimentation process yielded important results in order to know the behavior of the lactic strain for processes such as spray drying, in this context, the results found in the first phase are presented below:
|
a. Gas production |
b. Catalase |
Temperature (CFU/mL) |
|
|
Negative |
Negative |
35°C |
43°C |
|
|
|
3.0x1012 |
3.0x1010 |
In the fermentation kinetics, acceptable and optimal growth values were found for the lactic strain, which reached its exponential phase at time 4 (12 hours) with a value of 27.25 Ln CFU/mL, with sugar consumption values of 3.11 mg/L, protein production of 7.28 mg/L and lactic acid production of 1.26% (Figure 1).
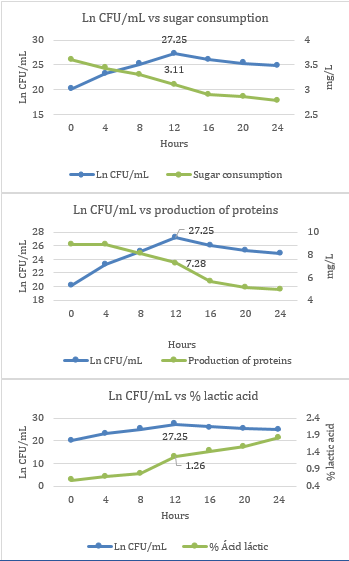
Figure 1 Kinetic parameters of L. lactis, sugar consumption, protein production and lactic acid production.
In this order of ideas, the negative production of gas in probiotic microorganisms corresponds as an important characteristic, since it has been evidenced that they control and prevent gastrointestinal disorders when frequently administered in the diet to the consumer and/or host.22 However, from the above mentioned and demonstrated in the food industry, there are some probiotic strains of interest in gas production, as in the case of the cheese and dairy industry for its application in the formation of "eyes" in cheeses and the foamy character of some fermented milks.23 Regarding resistance to different temperature levels, Amorocho24 mentions that the ability to maintain a viable bacterial concentration at temperatures between 2°C and 53°C has been demonstrated. For their part, Gouin25 and Sanchez et al.,26 found by subjecting Lactobacillus strains obtained from the intestinal tract of neonatal calves, to temperatures of 30°, 37° and 45°C, a favorable bacterial development, and where they also observed a higher yield capacity in viable cells between 9.3-10 log CFU/mL at a temperature of 37°C.
Vargas et al.,27 mention that the production of biomass from lactic acid microorganisms has acquired greater value and demand, taking into account its importance in the application in different processes in the food and pharmaceutical industry. In this regard, Jurado & Jarrín,28 in their research found a growth at 14 hours of 1.3 x 1013 CFU/mL in MRS medium, values of 0.806% in lactic acid, 1.24 mg/L of protein and 2.70 mg/L of sugar, for his part Rodríguez-González,29 observed when evaluating the kinetic behavior in MRS medium, in two isolated strains of L. plantarum F35 and F47, a growth of 9.5x108 CFU/mL at 15 h, and 3.0x109 CFU/mL at 24 h. In this order of ideas, Parra-Huertas,30 argues that for the performance in certain growth medium of these microorganisms the nutritional elements are relevant, especially, those elements that provide them with energy or that fulfill a role of energy source, in this way the author mentions, that the high nutritional requirement and the amount of energy that they can obtain by fermentation, condition the natural habitats that are favorable for the development of these bacteria, being the same: milk and products derived from it, the intestine and mucous membranes of humans and animals, as well as intact and decaying plants. They are microorganisms that are capable of fermenting monosaccharides or polysaccharides to transform them into acids such as lactic, citric, propionic, exopolysaccharides, bacteriocins, hydrogen peroxide, non-caloric sweeteners, vitamins, milk beverages, silage, formation of desirable flavors and aromas, cheese ripening, among others.
In the evaluation of the physicochemical parameters of the probiotic powder, optimal values were found, which show the ability of the microorganism to adapt, overcome and survive the different operating conditions of the spray drying process, thus, values were found for the variables of: viability (3x109 CFU/mL), Aw (0.401), humidity (5.15%), wettability (2:01 min), solubility (85%), hygroscopicity (0. 87) and microencapsulation efficiency (85%). The literature reporting information on L. lactis microencapsulated in the matrix proposed in the present research is limited, however, it has been reported by Salazar-Montoya et al.,31 a viability value of 8.12x1010 of an association of Lactococcus lactis subsp. lactis and L. lactis subsp. cremoris in a gellan gum matrix. Based on the above stated, according to authors such as Abd El-Salam & El-Shibiny,32 the great challenge in the application of microorganisms in food is to maintain viability during processing, storage and shelf life of the products, in the face of oxidative stress, temperature and acid-base changes to which they are in food matrices). Consequently, according to Martins et al.,33 the industrial use of probiotics depends on preservation methods using dehydration preservation methods to ensure better viability and cellular metabolic activity during processing and storage. Linked to the technique, morphology and capsule size were also evaluated, finding values between 680 nm and 13 µm for the strain under study (Figure 2). In this regard, Medina-Torres et al.,34 state that depending on the suspension and encapsulating agents used and the operating conditions, spray drying can produce a very fine powder (10-50µm) or large particles (2-3 mm), which is why it is a common technique for producing food encapsulates. In addition to the above it is important to mention that encapsulating agents also play an important role after the technique used to optimize the viability of the microorganisms, in this case, carbohydrate-derived agents were selected, and authors such as Huang et al.,35 report that carbohydrates are widely used materials for microencapsulation, due to their ability to bind to compounds, for their thermoprotective capacity, wall material, as well as their diversity and low cost.
In the evaluation tests of the probiotic powder under simulated gastrointestinal conditions, in the lactic strain under study, an important survival to these conditions was observed, overcoming the physiological enzymatic barriers found in the TGI. This result is possibly due to the thermal and wall protection of the microencapsulating material. In studies conducted by Gonzales-Cuello et al.,36 they found in a microencapsulated L. delbrueckii lactic strain subjected to simulated gastric juices, viability values of 4.56 log CFU/mL, Okuro et al.,37 found similar results where it was observed that microencapsulation of Lactobacillus acidophilus together with prebiotics (inulin and polydextrose) was able to protect cells from simulated gastric conditions. These results allow suggesting its use in probiotic dietary preparations aimed at improving technological parameters of products in the food industry, as well as to favor the host's health through its incorporation in the diet. Regarding the adhesion tests, a positive response was obtained (Figure 3), showing adherence of bacterial cells to the intestinal tissue; this result evidences the survival capacity of the lactic strain according to the information reported throughout the text. In turn, it is an important parameter when talking about the establishment of the lactic strain at intestinal level and expression of its probiotic potential.
Finally, in the determination of the inhibition capacity of the lactic strain on Salmonella typhimurium, significant differences were found among the treatments evaluated (P > 0.05). The halos formed in the different methods are presented in Figure 4. L. lactis presented the greatest inhibition size in the agar disk method, followed by the PADS method, and finally the well methods, the latter being similar to each other. Thus Mu, Tavella & Luo,38 mention that there are several characteristics for the selection of Lactobacillus as potential probiotics, including antimicrobial activity against various pathogens, ability to tolerate acid pH and survive in the GIT, adhesion to intestinal mucosal surfaces and inhibition of enteropathogen adhesion in the intestine. Roller,39 argues that, of all the antimicrobial substances produced by lactic acid bacteria, bacteriocins appear to be the most suitable from a technological point of view for use as food grade preservatives. In principle, their peptidic nature allows their degradation by digestive enzymes, thus being presumptively harmless to the consumer and his intestinal microbiota of occupation and, in some cases, their spectrum of action includes potential pathogens and spoilage agents associated with food (Bacillus cereus, Staphylococcus aureus, Listeria monocytogenes, Clostridium spp, among others). Finally, their physicochemical properties make them resistant to heat treatments and pH changes that foods undergo during manufacture and storage and, in addition, their small size allows diffusion in semisolid systems, typical of most food products. Within this context, Gonzáles-Toledo,40 state that L. lactis in a lactic acid battery with biopreservative properties, since some strains are capable of producing bacteriocins, among which nisin is the most notorious and widely used in the food industry. Moreover, it is the only bacteriocin that has been generally recognized as safe (GRAS) by the FDA-Food and Drug Administration and more than 50 countries.
According to the evidenced, it is established that the spray drying technique is an allied technique to favor the viability and survival of the lactic strain under study, in addition to the above, the preliminary experimentation showed a favorable behavior for the safe application of the technique. In all the inhibition methods established, an important inhibitory characteristic was found, however, the agar disc and PADS methods obtained a better performance with respect to the others. In this order of ideas, the lactic strain L. lactis is an important strategy in bioconservation and biocontrol for its application in different food matrices in future research.
The authors are grateful for the support and funding received from the Vicerrectoría de Investigación e Interacción Social (VIIS) of the Universidad de Nariño, and the research group PROBIOTEC-FORAPIS, attached to the same.
Research funded by Vicerrectoría de Investigación e interacción Social (VISS) - Universidad de Nariño, through Convocatoria de Investigación Docente 2021.
The authors declare that there are no conflicts of interest.

©2023 Jurado-Gámez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.