MOJ
eISSN: 2576-4519


Research Article Volume 4 Issue 5
1University of Manouba, Tunisia
2Faculty of Sciences of Tunis, University of Tunis El Manar, Tunisia
3Management Environment Responsible in Tanneries Mégisseries du Maghreb, Tunisia
Correspondence: Mohamed Neifar, LR Biotechnology and Bio-Geo Resources Valorization (LR11ES31), Higher Institute for Biotechnology, University of Manouba Biotechpole of Sidi Thabet, Sidi Thabet, Ariana, Tunisia, Tel 00216-70527882
Received: August 14, 2020 | Published: September 1, 2020
Citation: Ouertani R, Chouchane H, Mahjoubi M, et al. Feather degradation efficiency and hide dehairing ability of a new keratinolytic bacillus halotolerans strain, isolated from a tannery wastewater. MOJ App Bio Biomech. 2020;4(5):102-109. DOI: 10.15406/mojabb.2020.04.00143
Here, we report a new keratinolytic-producing bacterium identified as Bacillus halotolerans 4BC based on 16S rRNA gene sequencing. 4BC strain isolated from a tannery wastewater, showed proteolytic activity when grown on keratin azure, bovine hair and feather meal agar plates. B. halotolerans 4BC degraded almost 88% of chicken feathers after 10 days of cultivation in feather powder broth at pH 8 and 37°C. It was also efficiently able to degrade bovine hair keratin, despite the complexity of this substrate in comparison to feather keratin. The effects of different liquid to substrate ratios, inoculum sizes and incubation times on keratinase production were studied using response surface methodology to find the optimum conditions required for maximum B. halotolerans keratinase yield. The maximum keratinase yield (1059±53 mU/ml) was found under the following conditions: incubation period of 10 days, liquid/solid ratio of 5 and inoculum size of 2.3 % v/v). The crude enzyme exhibited a remarkable activity and stability under high temperature and alkaline conditions (pH 10 at 80°C). Additionally, the touch-visual and histological results demonstrated that the enzyme treated dehaired hides exhibit similar or improved characteristics without damaging the collagen layer, which makes the crude keratinase a potential effective and eco-friendly candidate for application in leather industry to avoid pollution problems associated with the use of chemicals.
Keywords: bacillus halotolerans, tannery wastewater, extremophilic keratinase, hide dehairing, feather degradation
Due to their extremely recalcitrant structures caused by the presence of disulfide bridges, hydrogen and hydrophobic bonds, keratins are resistant to environmental physical and chemical attacks as well as to proteolytic enzymes, such as trypsin, pepsin and papain.1,2 Keratin-rich wastes, mainly containing feathers and hairs, are generated in huge amounts by tanneries and agroindustries, and the accumulation of such residues leads to environmental problems.3 However, despite their recalcitrance, keratins can be efficiently hydrolyzed by keratinase-producing bacteria and fungi generally isolated from leather and poultry wastes.4–10 In this case, the Bacillus genus appears as efficient keratinolytic enzymes producers11–16 with potential applications in poultry, detergent, leather, cosmetic, biopolymer and pharmaceutical industries.2,19–19 Screening and discovery of extremophilic and extremotolerant keratinase-producing Bacillus strains from industrial wastewaters is a very promising environmentally‐friendly option for the discovery of novel green biocatalysts useful in poultry and leather processing industries. Accordingly, the present study aimed to identify and characterize a novel keratinase producer, the strain 4BC isolated from a local leather tannery (TMM, Tunisia). Optimisation of keratinase production in culture medium containing feathers was conducted using response surface methodology. The potential application of the enzyme for the dehairing of bovine hides in leather processing industry was also evaluated.
Substrates and chemicals
Chemicals and reagents were of the analytical grade. Bovine hides were kindly provided by the TMM leather tannery (Grombelia, Tunisia). Chicken feathers (whole feathers) were collected from chicken processing unit in Tunisia. Feathers were extensively washed in tap water to remove all the impurities. Feathers were dried at 60°C for 72 h and then chopped into fine pieces and ground. They were stored at 4°C under used.
Bacterial isolation, keratinase production and potential use in feather and hair degradation
Bacterial strains were isolated from TMM wastewater and screened for their ability to hydrolyze keratin in agar plates containing (g/L): chicken feather 10.0 or keratin azure 1.0, NaCl, 0.5; MgCl2•6H2O, 0.1; KH2PO4, 0.3; K2HPO4, 0.3; NH4Cl 0.5 and bacteriological agar, 17(pH 8).13 The plates were incubated at 37°C for 7 days. Bacterial colonies showing keratin hydrolysis zones were evaluated as positive keratinase producers. The selected efficient keratinase-producing bacterium was cultivated in 5-15mL nutrient broth medium (composition stated above). The production media, initially maintained at pH 8 and contained 1g of whole feather, were inoculated with 1-3% of Nutrient broth culture of O.D600≈0.6 served as inoculum. Inoculated production media were incubated at 37°C for 10 days with shaking rate of 180 rpm on a retoray incubator and enzyme production was checked after every 24h. The bacterial growth was estimated by determining the absorbance at 600 nm. The feather degradation yield was determined by the calculation of the difference in residual feather dry weight between abiotic control and the bacterial treated sample.20 For enzyme assays, the bacterial culture filtrates were centrifuged at 10.000 rpm for 15min and the supernatants were used as crude enzyme solutions.
Assay of proteolytic activities
Keratinase activity was determined using a modified protocol with chicken feather powder as a keratin substrate.21 The feather powder was suspended in 0.05 M Tris-HCl buffer (pH 10.0). The reaction mixtures containing 500 μl of the enzymatic extract and 500 μl of substrate (10 g/L) were incubated for 1h at 80°C. The enzymatic reaction was stopped by the addition of 400 μl of 10% trichloroacetic acid (TCA). After 15min incubation at 4°C, the reaction mixtures were then centrifuged at 10000 rpm for 15 min. The absorbance of the enzyme mixture was measured at 280 nm using an ultraviolet-visible spectrophotometer. One unit of keratinase activity was defined as the amount of enzyme required to release 1μmol of tyrosine per minute under the experimental assay conditions. Collagenase assay was carried out using Azocoll as a substrate.22 Azocoll was suspended in a 0.05 M Tris-HCl buffer (pH 8.0) containing 1 mM CaCl2 to a final concentration of 0.5% (w / v). Then, 100 µl of the enzyme extract and 100 µl of the Azocoll suspension were mixed with 800µl of Tris-HCl buffer and incubated at 37°C. After 1h incubation, the reaction was stopped by cooling the samples on ice for 5 min and then centrifuged at 10000 rpm for 20 min. The absorbance of enzymatic mixture was measured at 520 nm using an ultraviolet-visible spectrophotometer. One enzymatic unit is defined as the amount of enzyme required to convert 1μmol of substrate into product per minute.
Strain identification and phylogenetic analysis
The molecular identification of the keratinolytic bacterium 4BC was based on the sequencing of the 16S rDNA as described by Mahjoubi et al.23 The 16S rDNA sequences are compared to nucleotide sequences available in international databases NCBI using BLAST. The alignments of the nucleotide sequences and phylogenetic tree construction were performed by the software MEGA 6.06. Optimization of keratinase production in low cost media using a Box-Behnken Design (BBD). A 16 experiments Box-Behnken design (BBD) with three variables namely Liquid/solid ratio (X1), inoculum size (X2), and incubation time (X3), was carried out to establish the best experimental conditions for keratinase production (Table 1). The relationship between the response (keratinase production) and the three quantitative variables was modeled by the following polynomial model equation: Y = b0 + b1X1 + b2X2 + b3X3 + b11X21+ b22 X22 + b33 X23 + b12 X1X2 + b13 X1X3 + b23 X2X3; where Y represents the response, Xi and Xj are the levels of the independent variables and bi, bii and bij are the linear, quadratic and interactive coefficients, respectively. Statistical significance of the model and its parameters was determined at the 5% probability level (α =0.05) by analysis of variance. Three-dimensional response surface and contour plots were generated by the experimental design software NemrodW.24
No. exp. |
L/S Ratio |
Inoculum size |
Time |
Measured enzyme activity |
Estimated enzyme activity |
|
mL/g |
% |
(d) |
mU/ml |
mU/ml |
1 |
5.0 |
1.0 |
6.0 |
643.0 |
643.0 |
2 |
15.0 |
1.0 |
6.0 |
321.0 |
344.0 |
3 |
5.0 |
3.0 |
6.0 |
873.0 |
850.0 |
4 |
15.0 |
3.0 |
6.0 |
229.0 |
229.0 |
5 |
5.0 |
2.0 |
2.0 |
321.0 |
367.0 |
6 |
15.0 |
2.0 |
2.0 |
91.0 |
114.0 |
7 |
5.0 |
2.0 |
10.0 |
1057.0 |
1034.0 |
8 |
15.0 |
2.0 |
10.0 |
413.0 |
367.0 |
9 |
10.0 |
1.0 |
2.0 |
229.0 |
183.0 |
10 |
10.0 |
3.0 |
2.0 |
275.0 |
252.0 |
11 |
10.0 |
1.0 |
10.0 |
643.0 |
666.0 |
12 |
10.0 |
3.0 |
10.0 |
643.0 |
689.0 |
13 |
10.0 |
2.0 |
6.0 |
505.0 |
562.5 |
14 |
10.0 |
2.0 |
6.0 |
551.0 |
562.5 |
15 |
10.0 |
2.0 |
6.0 |
643.0 |
562.5 |
16 |
10.0 |
2.0 |
6.0 |
551.0 |
562.5 |
Table 1 Experimental conditions of the box-behnken design and the corresponding experimental and estimated 4BC keratinase production yields
Biochemical characterisation of bacterial keratinase
After ammonium sulphate precipitation (80% saturation) and dialysis, the effect of temperature on keratinase activity was assayed at different temperatures ranging from 30-100°C at pH of 8. The enzyme activity assays were carried out at 80°C over the pH range 4-9 using 0.05 M acetate buffer (pH 4 and 6), 0.05 M Tris-HCl buffer (pH 8) and 0.1 M Glycine-NaOH buffer (pH 10 and 12). The pH stability was determined by pre-incubating the enzyme in buffer solutions with different pH values for 2 h at 80 °C.
Dehairing of bovine hides and histological analysis
Bovine hide samples were soaked with three changes of water containing wetting and preservative agents until they are free from dirt and blood stains. Dehairing was performed as described by Khandelwal et al.25 Crude enzymatic extract (0.0Unit collagenase and 4.8 Unit keratinase per gramme of hide) was applied uniformly on the flesh side of each hide and left at ambient temperature (24±2°C) for 12hours. After enzyme addition, dehairing was performed manually with blunt knife. Pieces (5x5 cm) of dehairing /unhairing hides were fixed in 10 % formaldehyde solution for 24 hours to avoid autolysis. Sample (1x1 cm) pieces were dehydrated by immersion in ethanol baths of increasing degree (70-100) then treated in toluene baths and embedded in paraffin blocks. Sections of four-micron thickness of the embedded samples were cut using Leica RM2235 microtome and stained with hematoxylin and eosin to examine the histological features. These sections were examined under light Olympus UC90 microscope and photomicrographs were taken by using Cell Sens Entry software.
Isolation, identification and molecular phylogeny of a novel keratinolytic bacterium. Agar plate assays containing pure and complexe protein substrates were commonly used for the initial qualitative screening of microbial proteolytic activities.26 102 bacterial strains that were newly isolated from hides, skins and effluents samples from the TMM leather industry (Grombelia, Tunisia) were screened for keratinase production based on their clear zone formation patterns on keratin containing media at pH 8. The ratio of the clear zone diameter and that of the colony served as an index for the selection of keratinase-producing isolates. 4BC strain exhibited a high keratinase activity revealed by the clear proteolysis zone observed around the colonies after 7 days of incubation at 37°C (Figures 1A&1B). This isolate was also able to degrade efficiently whole feather or bovine hair during semi-solid state and submerged cultivations, respectively Figures 1C&1F, demonstrating that it could utilize the keratin based substrates as the only source of carbon and energy for bacterial growth. Indeed, it was observed that only 12±1 and 32±4% of the initial weights of chicken feather and bovine hair remained after 10 days of cultivation, respectively. The higher feather-biodegradation and dehairing abilities by keratinase producing 4BC isolate, in the preliminary experiments, confirm its promising potential application in biotechnological bioprocesses involving the dehairing of hides and the bioconversion of feather-rich wastes into valuable meal.
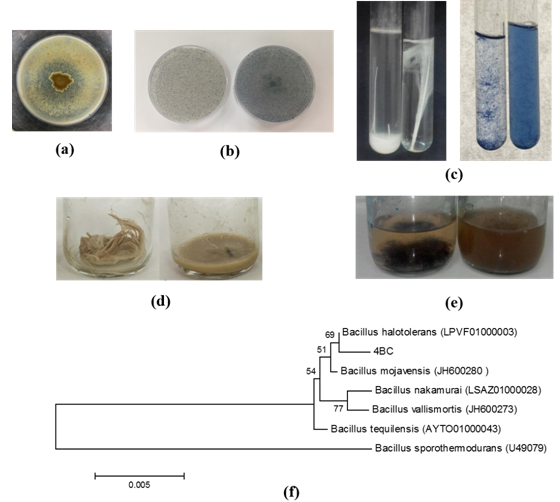
Figure 1 Keratinolytic activity detection during growth of B. halotolerans 4BC on: (a) Feather meal agar; (b) Keratin azure agar; (c) whole feather broth; (d) Keratin azure broth; (e) Solid state fermentation using feather as substrate; (f) bovin hair biodegradation by B. halotolerans 4BC and (g) Phylogenetic analysis of 16S rRNA gene sequence of bacterial isolate B. halotolerans strain 4BC based on 16S rDNA partial sequences. Phylogenetic dendrogram was evaluated by performing bootstrap analysis of 1000 data sets using MEGA 6.06 software. 16S rRNA sequence accession numbers of the reference strains are indicated in parentheses.
The isolated strain 4BC was subjected to molecular identification. The 16S rRNA gene sequence revealed a strong homology with those of several cultivated strains of Bacillus, reaching a maximum of 99% sequence identity. The nearest Bacillus strains identified by the BLAST analysis were the B. halotolerans (accession no. LPVF01000003), B. mojavensis (accession no. JH600280), and B. nakamurai (accession no. LSAZ01000028). Those sequences were imported into the MEGA software package version 4.1 and aligned. Phylogenetic trees were then constructed Figure 1G and the findings further confirmed that the 4BC strain (accession no. MF962877) was closely related to those of Bacillus halotolerans strains previously known as Brevibacterium halotolerans DSM8802.27 Different keratinolytic Bacillus strains have been previousely reported such as B. subtilis, B. cereus, B. polymyxa B. pumilus, B. tequilensis and B. thuringiensis 9,11,13,14,16,28,29 but this is the first report describing keratinase production by B. halotolerans.
Optimisation of keratinase production by B. halotolerans 4BC using responce surface methodology
The experimental BBD conditions of keratinase production by B. halotolerans 4BC were shown in Table 1. The obtained polynomial model is of the form: Y (keratinase, mU/ml)=562.5 - 230.0 X1 + 23.0 X2 + 230.0 X3- 11.5 X12 - 34.5 X22 - 80.5 X32- 80.5 X1X2- 103.5 X1X3-11.5 X2X3
Statistical analysis of the model Table 2 showed that the quadratic regression BBD model is highly significant with a computed F value of 29.3 implies the model is significant (P<F lower than 0.001). The value of coefficient of determination R2 is equal to 0.978, which indicated that model could explain 97.8 % of variability and unable to explain only 2.2 % of the total response variation. The adjusted R2 of 0.944 suggesting also a good fitness of BBD model for keratinase production. Table 3 highlighted the P values of each of the studied variables and their quadratic and interaction terms. The BBD model coefficients b0, b1, b3, b33, b12 and b13 are significant whereas b2, b11, b22, and b23 are not significant. The interaction effects of variables on keratinase production by B. halotolerans 4BC were studied by plotting the three-dimensional response surfaces with the vertical axis representing enzyme production and two horizontal axes representing the coded levels of two independent variables, while keeping the other variable at its central level (0) (Figure 2).
Source of variation |
Sum of squares |
Degrees of freedom |
Mean square |
Ratio |
Significance |
Regression |
951142 |
9 |
105682 |
29.2358 |
*** |
Residuals |
21689.0 |
6 |
3614.83 |
|
|
Validity |
11638.0 |
3 |
3879.33 |
1.1579 |
45.3% |
Error |
10051.0 |
3 |
3350.33 |
|
|
Total |
972831 |
15 |
|
|
|
Table 2 ANOVA for the response surface quadratic model for 4BC keratinase production***Significant at the level of 99.9 %
Nom |
Coefficient |
F.Inflation |
Stand. Dev. |
t.exp. |
Signif. % |
b0 |
562.5 |
|
30.1 |
18.71 |
*** |
b1 |
-230.0 |
1.00 |
21.3 |
-10.82 |
*** |
b2 |
23.0 |
1.00 |
21.3 |
1.08 |
32.2% |
b3 |
230.0 |
1.00 |
21.3 |
10.82 |
*** |
b11 |
-11.5 |
1.00 |
30.1 |
-0.38 |
71.4% |
b22 |
-34.5 |
1.00 |
30.1 |
-1.15 |
29.5% |
b33 |
-80.5 |
1.00 |
30.1 |
-2.68 |
* |
b12 |
-80.5 |
1.00 |
30.1 |
-2.68 |
* |
b13 |
-103.5 |
1.00 |
30.1 |
-3.44 |
* |
b23 |
-11.5 |
1.00 |
30.1 |
-0.38 |
71.4% |
Table 3 Estimated effect, regression coefficient, and corresponding t and P values for 4BC keratinase production in Box-Behnken design experiments (∗∗∗), significant at the level 99.9%; (∗∗), significant at the level 99%; (∗), significant at the level 95%; NS, Non-Significant
The effect of incubation period on 4BC keratinase production was illustred in Figures 2A&2C. As incubation period was increased from 2 to 10 days, the enzyme activity was found to increase and the maximum keratinase production was found at an incubation time of 10 days. In spore-forming bacteria, proteolytic enzyme production was often associated with the late logarithmic growth phase or the beginning of sporulation which occurred after depletion of easily available nutrients.28 Hence 10 days of incubation period was found to be the optimum incubation period. The effect of L/S ratio on B. halotolerans keratinase production was shown in Figures 2A&2B. As L/S was increased from 5 to 15 (v/w), the keratinase production by 4BC strain was found to decrease and a maximum enzyme production was found at L/S = 5 v/w. Further decrease in L/S ratio decreases the bacterial growth (data not shown). Many previous studies showed that complete degrading of chicken feathers with keratinolytic bacteria was only achieved with low substrate concentrations.30–32 Hence 20 (% w/v) was found to be the optimum substrate concentration. The same trend was obtained for the study of the effect of inoculum size on B. halotolerans keratinase production and the results are shown in Figures 2B&2C. As inoculum size was increased from 1 to 3 (% v/v), the keratinase activity was found to increase and a maximum enzyme production was found at 2.3 (%w/v). Further increase in substrate concentration decreases the keratinase activity. It was observed that inoculum size and L/S ratio had a strong interactive effect on keratinase production by B. halotolerans 4BC. The negative interaction between the two factors showed that there is a decrease in enzyme production with initial increase of inoculum size and L/S ratio; however, high levels of these two factors had inhibitory effects.
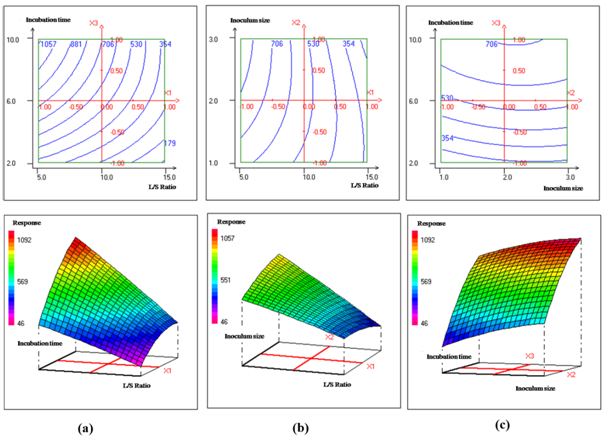
Figure 2 Contour and response surface plots of keratinases production by B. halotolerans 4BC as a function of: (a) L/S Ratio (X1) and Incubation time (X3) levels at midlevel of Inocumlum size (2%); (b) L/S Ratio (X1) and Inocumlum size (X2) levels at midlevel of Incubation Time (6 Days); (c) Inocumlum size (X2) and Incubation time (X3) levels at midlevel of L/S Ratio (10).
The optimum levels for liquid to solid ratio, inoculum size and incubation time were predicted by NemrodW software as 5 v/w, 2.3% v/v and 10 days, respectively. Under these conditions, a maximum keratinase production of 1059±53 mU/ml was obtained. This predicted value was in good agreement with experimental data (1071±56mU/ml). Biochemical characterization of crude keratinase and potential application in hide dehairing. Figure 3A shows that B. halotolerans 4BC displayed keratinase activity over a broad range of pH (4-12), with an optimum at pH 10. The relative activities at pH 4, 6, 8 and 12 were 50.6 %, 67.8 %, 76 % and 56.5 %, respectively. The pH stability profile indicated that the enzyme was highly stable in the pH range between 10 and 12 (Figure 3C). It retained 92.8 %, and 92.1 % of their activity at pH 10 after 1 and 2 h incubation at 80 °C, respectively. These findings indicated that the thermoactivity and thermostability of B. halotolerans keratinase were higher than those previously reported for several other bacterial keratinases8,21,31 All these properties suggest that B. halotolerans keratinase may be useful candidate in industrial applications. The incubation of B. halotolerans keratinase with bovine hides for 12 h at 37°C is efficiently able to remove all the hair from bovine hides without observable damage on their collagen (Figure 4). The enzymatic treated hides displayed clean hair pores and intact grain structures. These results provided evidence that B. halotolerans keratinase could efficiently accomplish the whole process of hide dehairing in leather industry. As this process is generally carried out under alkaline conditions (pH values ranging between 8 and 10), the use of extremozyme from B. halotolerans seems to be very promising in term of catalytic performance and hair removal capacity.
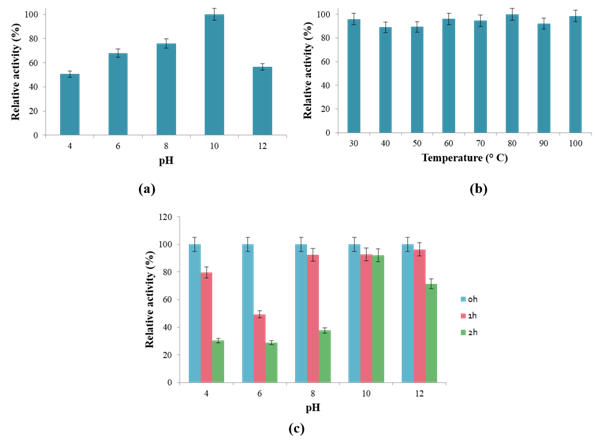
Figure 3 Effects of pH (a) and temperature (b) on the activity of B. halotolerans keratinase. Stability of B. halotolerans keratinase at different pHs at 80°C (c).
Figure 4B macroscopically shows that keratinase dehaired was clean, whitish and had smooth feel due to epidermis removal. Hair obtained was also intact. Reported literature indicates that diffusion of enzymes to proteolytic sites is a crucial fact. The penetration of the enzyme can bring about the destruction of proteins located in the vicinity of the cells of the epidermis and the keratin layer inside the hair follicles. The optical micrograph of the stained cross-section of cow hide Figure 4D confirms that complete removal of epidermal and keratin layer from hide was observed when dehairing was performed using the B. halotolerans 4BC keratinase. The microscopic structure of cross-section clearly demonstates that keratinase dehairing is very effective with respect to the extent of removal of epidermis, keratin layer, hair shaft, and follicles (Figure 4C&4D). Besides, keratinase dehairing leads to complete and uniform collagen fiber in the dermis region and empty hair follicles. Few similar results on enzymatically dehaired pelts have been reported by other researchers.25,33 To the best of our knowledge, this work is the first reference to Bacillus halotolerans as a producer of extremotolerant keratinase with a promising potential in the biodegradation of checken feather wastes and dehairing of bovine hides. All these properties suggest that B. halotolerans keratinase may be useful candidate in industrial applications compared to other keratinase-producing Bacillus strains (Table 4).34–41

Figure 4 Histological analysis of bovine hide treated with the crude enzymatic extract of B. halotolerans 4BC. (a) Control, water-treated hide; (b) Hide after a 12-h treatment with 4BC keratinase (4.8 U/g of hide).
Keratinase producing strain |
Range of enzyme activity |
Keratin substrate |
Biotechnological and industrial application |
Refereces |
Bacillus altitudinis RBDV1 |
35 - 95 °C |
chicken-feather keratin |
a promising condidate for enzymatic, processing of keratinous wastes |
Pawar et al. 2018 |
Bacillus subtilis DP1 |
pH 8 - 12; 20 - 50 °C |
chicken-feather keratin, rabbit hair |
a potential utility in cosmetic formulation |
Sanghvi et al. 2016 |
Bacillus licheniformis ALW1 |
pH 7 - 9; 50 - 60 °C |
chicken-feather keratin |
potential in feather waste biodegradation and other biological applications |
Abdel-Fattah et al. 2018 |
Bacillus subtilis BF11and Bacillus cereus BF21 |
|
chicken-feather keratin |
role in producing food and feed supplements with high nutritive value |
Lakshmi et al 2012 |
Bacillus thuringiensis TS2 |
pH 10; 50 °C |
active on natural and synthetic proteolytic substrates but inactive against collagen |
potential in proteinaceous waste management and hide dehairing |
Sivakumar et al. 2012 |
Bacillus safensis LAU 13 |
pH 5 - 11; 30 - 60 °C |
chicken-feather keratin |
potential in the biodegradation of feather wastes, destaining of blood-stained fabric and dehairing of animal skin |
Lateef et al. 2014 |
Bacillus weihenstephanesis PKD5 |
pH 5 - 8; 37 °C |
keratin powder |
milk clotting potential for cheese making |
Sahoo et al. 2015 |
Bacillus tequilensis strain Q7 |
pH 4.5 - 10; 25 - 50 °C. |
feather-meal, the most degraded (100%), followed by chicken feather (85%), rabbit hair (76%), goat hair (64%), bovine hair (43%), and sheep wool (11%). |
Enhancing enzymatic process for animal hide bating in the leather processing industry |
Jaouadi et al. 2015 |
Bacillus pumilus ZED17 |
pH 7 - 10; 25 - 40 °C, |
feather, meat, gelatin and casein |
dehairing of buffalo hide, waste valorization and conversion to biomass |
Talebi et al. 2013 |
Bacillus cereus KB043 |
pH 6-9; 25 - 45 °C |
feather wastes |
useful in management of poultry wastes |
Nagal and Jain 2010 |
Bacillus megaterium KB008 |
pH 7 - 10 ; 37 - 45 °C |
chicken-feather keratin |
potential application in medicine and agriculture |
|
Bacillus amyloliquefaciens MA20 |
pH 6 - 12 ; 4 – 50 °C |
wool keratin |
potential application in wool industry |
Hassan et al. 2013 |
Bacillus Halotolerans 4BC |
pH 4 - 12 ; 30 – 100 °C |
chicken-feather, bovine hair, human hair, casein, keratin azure |
biodegradation of feather wastes, dehairing of animal skin in leather industry, treatment of tannery wastewater |
This work |
Table 4 Some keratinase-producing Bacillus strains revealing biotechnological and industrial potentialities
A novel B. halotolerans 4BC, isolated from the effluent of TMM leather industry, produced extracellular proteases and efficiently degraded insoluble keratin during cultivation with whole feathers or bovine hairs as the only source of carbon and energy. The maximum keratinase production (1059±53 mU/mL) by 4BC strain was achieved after 10 days of incubation at 37°C and pH 8 at 180 rpm in feather based medium with economic liquid to solid ratio of 5 (v/w) and inoculum size of 2.3 (% v/v). In addition, the enzyme was found active over a wide range of pH and was relatively stable up to 70°C. In this respect, and showing higher feather-biodegradation and dehairing abilities, B. halotolerans keratinase may be considered a potential promising candidate for future application in biotechnological bioprocesses involving the dehairing of hides and the biodegradation of keratinous wastes generated by agroindustrial processing especially waste feathers.
None.
The authors declare, that there is no conflict of interest.
None.

©2020 Ouertani, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.