Journal of
eISSN: 2373-6410


Case Report Volume 1 Issue 5
1Department of Neurology, Tallahassee Neurobalance Center, USA
2Department of Ophtalmology, Medical University of Gdansk, Poland
Correspondence: J. Lucas Koberda, Department of Neurology, Tallahassee NeuroBalance Center, Tallahassee, FL 3239, USA, Tel 850-877-2802, Fax 850-222-1383
Received: June 25, 2014 | Published: September 11, 2014
Citation: Koberda JL, Stodolska-Koberda U. Z-score LORETA neurofeedback as a potential rehabilitation modality in patients with CVA. J Neurol Stroke. 2014;1(5):155-158 DOI: 10.15406/jnsk.2014.01.00029
This is multi-case presentation describing promising rehabilitation results of Z-score LORETA neurofeedback therapy of patients suffering from prior stroke. Potential benefits include improved cognitive function and motor performance.
Keywords: CVA, LORETA, Neurofeedback, Rehabilitation, Z-score, Imaging
NFB, Neurofeedback; LORETA, Low Resolution Electromagnetic Tomography Analysis; CVA, Cerebrovascular Accident; MCA, Middle Cerebral Artery; ICA, Internal Carotid Artery; GCS, Global Cognitive Score; IPS, Information Processing Speed; BA, Brodmann’s Area
EEG biofeedback also called neurofeedback (NFB) has been shown to be a promising modality in neurorehabilitation including stroke.1-4 Recent progress in computer technology and development of newer imaging and NFB techniques including low resolution electromagnetic tomography analysis (LORETA) as well as Z-score NFB prompted me to apply this therapy in stroke patients. The potential advantage of LORETA Z-score NFB is ability to achieve even faster results than standard one or two channel neurotherapy.5,6 Deep structures such as the anterior cingulate cortex,7 insula and mesial temporal lobes8 can be correctly localized with LORETA. In Z-score biofeedback real-time comparisons to an age matched reference population of healthy or normal subjects are used as a guide or “compass” to increase specificity and provide a uniform direction and threshold for the biofeedback process.9 Prior to Z score biofeedback clinicians had to guess about what threshold to set for a given frequency or location to trigger the feedback signal or reinforcer signal.9
Z score biofeedback greatly simplifies and standardizes EEG biofeedback by reducing many different metrics (absolute power, relative power, ratios, coherence, phase) to a single or common metric of the Z score or a standard deviation with respect to the EEG from a group of age matched healthy normal subjects.9
Surface Z scores improve specificity by isolating dysregulated locations and rhythms, especially when using the Laplacian transform and LORETA Z scores are even more specific. For example, LORETA Z score biofeedback often also produces results in one 20 minute session because EEG source localization has accuracies of about 1 cm to 3 cm and thus is much more specific than is surface EEG.9
In Z-Score NFB, a real-time comparison to an age-matched population of healthy subjects is used for data acquisition, simplifying protocol generation and allowing clinicians to target modules and hubs that indicate dysregulation and instability in networks related to symptoms. Z-score NFB increases specificity in operant conditioning, providing a guide that links extreme Z-score outliers to symptoms, and then reinforcing Z-score shifts toward states of greater homeostasis and stability. The goal is increased efficiency of information processing in brain networks related to the patient’s symptoms.10
A recently introduced method called Low Resolution Electromagnetic Tomography (LORETA) Z-score NFB is capable of targeting specific dysregulated anatomical structures, many of which are in deep cortical locations.11-13 For example, the insula and anterior cingulate have been identified as potential NFB target sites for improving pain control in patients who display electrical dysregulation of these areas.12
In my clinic, we have completed Z-score LORETA therapy with more than 250 patients with different neuropsychiatric conditions including five patients suffering from stroke. One of the patients who was diagnosed with occipital cerebrovascular accident (CVA) and complained of visual problems due to homonymous hemianopia completed only 3 NFB sessions and reported subjective improvement of his vision. However, no follow up visual fields study was completed. In this paper, I will present four patients with their rehabilitation outcomes that completed longer courses of Z-score LORETA NFB due to ischemic or hemorrhagic stroke.
Deymed Truscan 32 (Deymed Diagnostic, Payette, ID) EEG equipment was combined with Neuroguide (Applied Neuroscience, Inc.) software. The NFB protocol included surface and LORETA (NF1/NFB2) feedback, in an alternating protocol while focusing on a symptom checklist.
A commercially available computerized neurocognitive testing battery was used for the initial and follow up assessments of some patients (NeuroTrax Corp, Bellaire, TX). NeuroTrax Corporation cognitive testing is a computerized neuropsychological assessment where the patient is compared to age and education matched healthy controls where mean is 100 and the standard deviation is 15. QEEG analysis was completed using commercially available Neuroguide software (Applied Neuroscience, Inc.) and previously recorded 19 channel digital EEG.
Approximately 1-3 minutes of artifact-free eyes closed EEG segments were selected after previously recording EEG with the Deymed, Truscan 32, (Deymed Diagnostic, Payette, ID) and subjected to further QEEG analysis. NFB therapy consisted of 30-minute sessions once or twice a week using auditory and/or visual feedback.
To localize the cortical sources of scalp EEG activity we used low resolution electromagnetic tomography analysis.14 The low resolution electromagnetic tomography analysis (LORETA) method is a discrete, three-dimensional (3D) distributed, linear, inverse solution. The smoothing constraints in LORETA endows the tomography with the property of low localization errors to test point sources, albeit with low spatial resolution (i.e. neighboring neuronal sources will be highly correlated.14 The LORETA inverse solution corresponds to the 3D distribution of electrical neuronal activity that features a maximum similarity (i.e. maximum synchronization), in term of orientation and strength, between neuronal populations in adjacent voxels. The imaging is therefore particularly tuned towards synchronized brain activities as they occur, e.g. in spreading oscillatory activations. Since LORETA takes explicitly into account that scalp electric potentials are determined up to an arbitrary additive constant, the final LORETA solution is independent of the electrical reference used. Deep structures such as the anterior cingulate cortex,7 insula and mesial temporal lobes8 can be correctly localized with LORETA.
Case 1
Fifty eight year old male with a history of prior CVA (7 years ago) with residual right hemiparesis and mostly expressive aphasia. Prior MRI showed left middle cerebral artery (MCA) infarct and MRA revealed left internal carotid artery (ICA) occlusion. QEEG showed marked increase in frontal delta and theta power. Patient completed 5 sessions of LORETA NFB with improvement of his speech and weakness noticeable after second session (after NFB able to speak sentences-before only single words; before NFB -unable to extend and flex fingers of his right hand-after NFB able to both extend and flex). Patient completed only 5 sessions due to his insurance termination.
Case 2
Forty three year old male with a history of cardiomyopathy and atrial fibrillation was diagnosed with two CVA’s (one 2 years prior his visit) with residual right sided weakness with cognitive problems and second CVA 6 month prior (left hemiparesis due to right MCA stroke). CVA was confirmed by MRI. QEEG showed mild increase in the left frontal and temporal delta power and marked hyper-coherence. Cognitive computerized testing (NeuroTrax) showed evidence of cognitive dysfunction including global cognitive score (GCS) of 88.4 with low score on information processing speed (IPS)-76.2 and verbal function-62 (Table 1).
Number of NFB sessions |
0 |
10 |
20 |
Global Cognitive Score |
88.4 |
92.8 |
98.6 |
Memory |
98.8 |
91.3 |
102.4 |
Executive Function |
94.4 |
91 |
102.8 |
Attention |
94.3 |
85.5 |
91.7 |
Information Processing Speed |
76.2 |
82 |
76.6 |
Visual Spatial |
100.7 |
95.9 |
105.4 |
Verbal Function |
62 |
99.9 |
99.9 |
Motor Skills |
92.2 |
103.9 |
111.2 |
Table 1 43 year old male with CVA- computerized cognitive testing (NeuroTrax) before and after 10 and 20 sessions of NFB
This patient was interested in NFB therapy for improvement of his cognitive function. After 10 NFB sessions another cognitive testing was completed which showed an improvement of GCS-92.8 as well as IPS-82 and verbal function-99.9. In addition, an improvement in motor skills (finger tapping) was recorded-103.9. After completion of another 10 sessions of NFB (total of 20) further raise in GCS was noted. Patient also reported marked improvement in his subjective perception of cognitive performance describing this as feeling “sharper”. Repeated (after NFB) QEEG showed improvement in coherence.
Case 3
Fifty seven year old male was seen in my office with interest of participation in NFB therapy. He suffered hemorrhagic stroke 4 years earlier, which left him with right hemiparesis, expressive aphasia and cognitive problems including short term memory. CT of the brain at the time of bleeding showed Parenchymal hemorrhage (5.0x 3.3 cm) deep within the left cerebral hemisphere at the left basal ganglia with surrounding edema.
Initial computerized cognitive testing before NFB showed low GCS-89.2 including memory 84, and low verbal-66.5 and motor functions-55.5. QEEG showed increased frontal and temporal delta and theta power (Figure 1a). LORETA revealed several areas of electrical dysregulation including the left temporal lobe Brodmann’s area (BA)-20 and 28 (Figure 1b). After completion of 10 NFB sessions patient reported some improvement in his right facial weakness. Repeated cognitive testing showed marked improvement of his cognitive testing including GCS-104, memory-95.6, verbal function-66.5 and motor movements-106.8 (Table 2). In addition, a correction of QEEG abnormalities was also noted after NFB therapy. Patient has completed 16 NFB sessions so far.
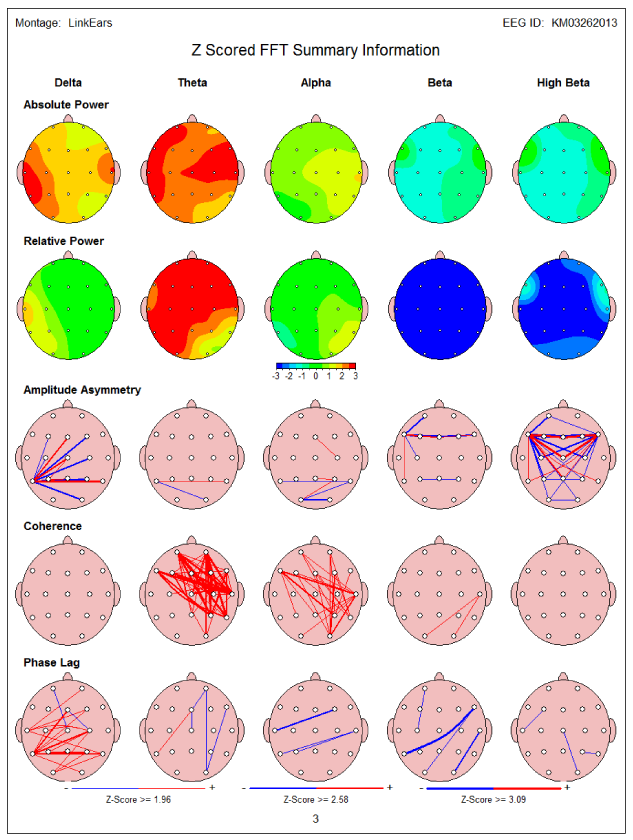
Figure 1a 57 year old with hemorrhagic stroke-QEEG showed increased frontal and temporal delta and theta power (in red).
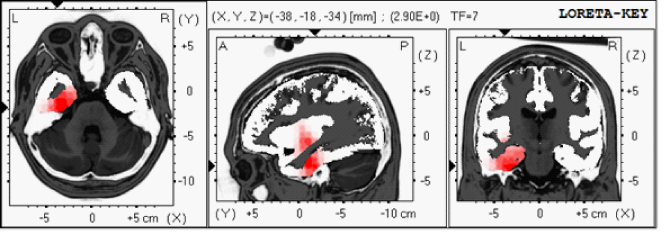
Figure 1b 57 year old with hemorrhagic stroke- LORETA revealed several areas of electrical dysregulation including the left temporal lobe Brodmann’s area (BA)-20 and 28.
Number of NFB Sessions |
0 |
10 |
Global Cognitive Score |
89.2 |
104 |
Memory |
84 |
95.6 |
Executive Function |
106.6 |
105 |
Attention |
106.6 |
107.7 |
Information Processing Speed |
96 |
108 |
Visual Spatial |
108.9 |
113.6 |
Verbal Function |
66.5 |
91.2 |
Motor Skills |
55.5 |
106.8 |
Table 2 57 year old with hemorrhagic stroke-computerized cognitive testing (NeuroTrax) before and after 10 sessions of NFB
Case 4
Sixty nine year old male came for rehabilitation with LORETA NFB after suffering from CVA (4 months earlier) (Table 3). During CVA he complained of confusion with memory problems, left sided numbness associated with pain and visual and spatial problems. MRI of the brain showed evidence of the right thalamic and temporal/hippocampal CVA and occlusion of occipital cerebral artery. QEEG showed increased right temporal delta and theta power (Figure 2A). LORETA showed several areas of electrical dysregulation including right temporal lobe BA-36 and cingulate gyrus BA-24 (Figure 2B).
Number of NFB Sessions |
0 |
10 |
Global Cognitive Score |
83.6 |
94.8 |
Memory |
85 |
99.7 |
Executive Function |
87.5 |
103.9 |
Attention |
80.2 |
94.2 |
Information Processing Speed |
74.4 |
84.3 |
Visual Spatial |
85.8 |
99.3 |
Verbal Function |
86.1 |
83.7 |
Motor Skills |
86 |
98.4 |
Table 3 69 year old with prior CVA- computerized cognitive testing (NeuroTrax) before and after 10 sessions of NFB
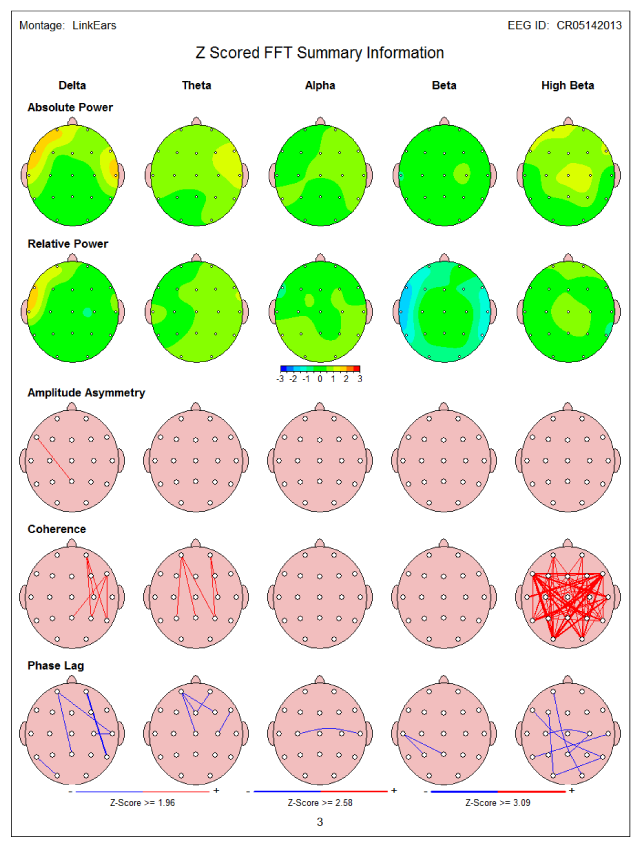
Figure 2a 69 year old with prior CVA- QEEG showed increased right temporal delta and theta power (in yellow).
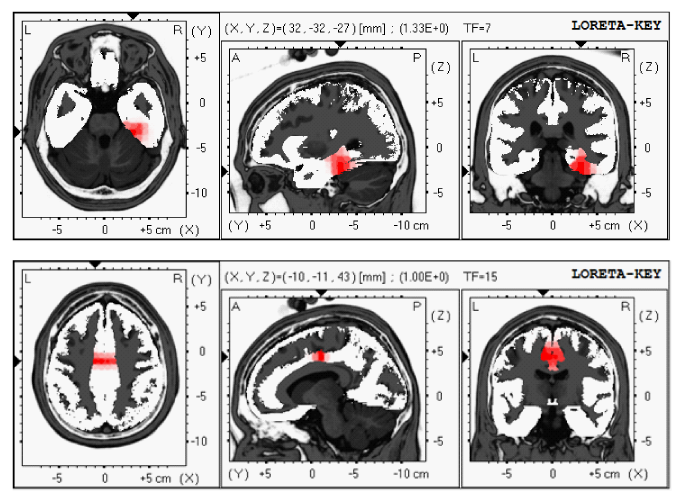
Figure 2b LORETA showed several areas of electrical dysregulation including right temporal lobe BA-36 and cingulate gyrus BA-24 (in red).
Initial (before NFB) NeuroTrax showed low cognitive score GCS-83.6 with low memory score-85.0 and IPS-74.4. After four sessions of NFB the patient reported some improvement of his cognitive symptoms. After 10 sessions repeated cognitive testing showed improvement in cognitive functions GCS-94.8 including memory-99.7, IPS-84.3 as well as improved other cognitive functions. In addition, a correction of QEEG/LORETA abnormalities was noted after NFB therapy completion.
This paper shows promising rehabilitation results of an initial group of patients who suffered from cerebrovascular accidents using LORETA Z-score NFB therapy. All reported patients indicated the subjective improvement of their symptoms. In addition, follow up objective computerized testing confirmed the cognitive enhancement after completion of neurotherapy. An improved speech and motor function were also reported by above stroke patients. Similar findings of neurological recovery were recently described by Rayegani et al.,1 where patients in the neurofeedback and EMG-biofeedback groups showed hand function improvement similar to conventional occupational therapy. The most likely explanation of positive role of NFB in CVA patients may be attributed to its neuroplastic effect. Ghaziri et al.15 from University of Montreal group have recently reported in randomized study that NFB training induces changes in white and gray matter. These changes were observed only in the experimental group. Based on this research the assumption can be made that NFB therapy may create new connections between parts of the brain affected by CVA. Longer follow up study with a control group (in order to reduce a placebo effect) is planned to evaluate likelihood of sustained effect of this rehabilitation modality. Our group previously reported successful cognitive enhancement of patients with autistic spectrum disorder,16 static memory problems attention deficit hyperactive disorder17 as well as Alzheimer’s disease.11 We found that this type of NFB is also effective in pain amelioration in patients suffering from neuropathic pain, headaches and lower back pain. The pain reduction was able to be achieved by correcting the areas of electrical dysregulation in the insular cortex and cingulate cortex.12 Similar beneficial results were achieved with patients suffering from medically refractory epilepsy13 as well as patients suffering from anxiety and depression.18
This paper shows marked improvement in both cognitive and motor functions of patients suffering from CVA with use of LORETA Z-score NFB. Further studies with larger number of patients and control group may be required to evaluate full rehabilitation potential of this technology in stroke patients.
None.
None.

©2014 Koberda, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.