Journal of
eISSN: 2373-6410


Case Report Volume 5 Issue 3
Department of Pediatrics, King Saud University, Riyadh, Saudi Arabia
Correspondence: Amal Y Kentab, Assistant Professor & Consultant Pediatric Neurologist, Division of Pediatric Neurology, Department of Pediatrics (39), College of Medicine & King Khalid University Hospital, King Saud University, P.O. Box 2925, Riyadh 11461, Saudi Arabia, Fax +966(1) 469-1512
Received: October 31, 2016 | Published: November 23, 2016
Citation: Kentab AY (2016) Unilateral Basal Ganglia Infarction in a Neonate Born to a Mother with Gestational Diabetes: Case Report. J Neurol Stroke 5(3): 00180. DOI: DOI: 10.15406/jnsk.2016.05.00180
Cerebral infarcts are an important cause of neonatal convulsions. Perinatal arterial ischemic stroke (PAIS) is a cerebrovascular event occurring during fetal or neonatal life, before 28 days after birth. It has an estimated incidence of one in 2300 to 5000 live births based on the consensus of the National Institute of Neurological Disorders and Stroke.1 Over 80% of cases involve the middle cerebral artery or its branches.2 It is an under- recognized cause of significant long - term disabilities, including hemiplegic cerebral palsy, epilepsy, cognitive delays and behavioral impairments.3 Perinatal BG stroke is uncommon, but the true incidence is unknown. The author report the etiologic factors, clinical and neuroradiologic findings of full term neonate born to a mother with gestational diabetes who presented with right - sided clonicneonatal convulsions and had an isolated unilateral basal ganglia (BG) stroke.
Keywords: Perinatal stroke, Risk factors, Gestational diabetes, Neonatal convulsion
Perinatal stroke may be defined as an acute neurologic syndrome with chronic sequelae due to cerebral injury of vascular origin occurring between 20 weeks gestation and 28 days postnatal life, confirmed by neuroimaging or neuropathological studies.1 These disorders include focal cerebral injury due to arterial ischemic stroke, cerebral venous thrombosis, and primary intracerebral hemorrhage. PAIS is the most frequent type of pediatric stroke, and account for 70 percent of acute symptomatic perinatal stroke.4 It is probably related to perinatal activation of coagulation mechanisms, affecting fetal and neonatal thrombogenesis during the critical perinatal period.5
Two proposed hypothesis for cerebral embolus in PAIS. One placenta-embolic hypothesis where an embolus, originating from the placenta reach the brain through the foramen ovale, and most of the established risk factors are indeed either determinates or biomarkers of vasculo-placental pathology. The second is the traumatic theory where injury to the cervico-cerebral arteries, giving rise to thrombus/embolus during the birth process. Both theories are supported by a few, but well- analysed pathological or arteriographic reports.3 This case report describe the clinical, and radiological features of a newborn with an isolated unilateral basal ganglia (BG) stroke, discuss the associated potential risk factors.
A male infant was born at term to 39 year old, gravida 7, para 6 mother with gestational diabetes mellitus (GD) on insulin , L- thyroxin for hypothyroidism , and vitamin D supplement for low vitamin D level 20.67 nmol/l ( NR 75- 250 ). GD was managed initially with diet restriction, and shifted to regular insulin and a regular insulin sliding scale in the last two months. However, glucose control was suboptimal, as evidenced by her elevated hemoglobin A1c level before delivery (8.7 % [normal: 6.4%]). Antenatal booking screen showed negative prenatal toxicology tests, and normal fetal ultrasound with no polyhydramnios, placental abnormalities or fetal anomalies. On arrival to our hospital her random blood glucose level was 238 mg/dl (normal:_200 mg/dL), and serum laboratory tests were normal with no evidence of metabolic derangements. Fetal scalp PH, and fetal heart rate monitoring revealed normal results. The baby was delivered by spontaneous vaginal delivery with birth weight of 3200 g and Apgar score of 8,9 at 1,5 minutes, respectively. His serum glucose was normal, and there was no immediate neonatal problems. He was discharged on the 2nd day in good condition. The baby presented to our hospital on the 3rd day of life with repetitive rhythmic, low-amplitude focal jerky movements involving the right upper and lower extremities associated with intermittent blink of right eye, right facial twitching, and minimal facial duskiness. These were consistent with seizures. He had low grade fever (not measured), and feeding difficulty at home with no reported lethargy or apnea. On clinical examination he washypoactive, normcephalic, icteric, mildly dehydrated, and had depressed newborn reflexes. The cranial nerve examination was normal. The left upper and lower limbs showed normal tone, power, and reflexes. Reduced spontaneous movements of the right arm.The right side tone was decreased in both the upper and lower limbs. The power was grade 3 in the right arm and grade 4 in the right leg. The deep tendon reflexes over the right knee and ankle were brisk. The plantar reflex was equivocal bilaterally. His chest, cardiovascular, and abdominal examinations were normal. On admission, as a serum glucose level was normal, a sepsis workup including cerebrospinal fluid (CSF) analysis was initiated and intravenous fluid hydration with antibiotics were prescribed, although his initial white blood cell counts were not suggestive of infection and the initial blood culture , CSF culture as well as PCR for herpes simplex type 1, ultimately returned a negative results. Transfontanell ultrasound (US) was normal, while brain computed tomography (CT) scan showed hypodense lesion at the left caudate and lentiform nuclei with no hemorrhage, calcification or hydrocephalus. The seizures were aborted after phenobarbitone loading, and maintenance. However, these events, were not associated with epileptiform activity on electroencephalography (EEG). Laboratory testing showed a white blood cell count of 8900/mm³ with 55% neutrophils, 30 % lymphocytes, and 14 % monocytes; hemoglobin 17.4 g/dL; and platelets 304 000/mm3. Total bilirubin high 139 umol/l (NR 3-17), direct bilirubin normal 5 umol/l (NR 0-5), consistent with indirect hyperbilirubinemia. Bone profile showed calcium 1.88 mmol/l ( NR 2.2 - 2.7) low, corrected calcium 2.1 mmol/l (NR 2.2 - 2.7), magnesium 0.8 mmol/l (NR 0.7-1.1), phosphorus 2.27 mmol/l (NR 1.45-2.91) with normal alkaline phosphatase. Liver function showed high aspartate aminotransferase (AST) 43 u/l (NR 12-37), and gamma glutamyltranspeptidase ( GGT ) 227 u/l (NR 15-85), with normal total protein and albumin. The blood urea nitrogen, electrolytes, lipid profile, hemoglobin electrophoresis, coagulation profile, homocysteine level, the prothrombin G 2010 mutation, Factor V Leiden mutation, anti-cardiolipin antibodies, double-stranded DNA antibodies, antinuclear antibodies (ANA) , metabolic including serum ammonia, lactate, venous blood gas, serum tandem amino acid assay, and urinary organic acid were all normal. Protein C, S, anti-thrombin III assays activity levels were low initially 61% (NR 65-140), 27% (NR 70 - 140), 75% (NR 80-120), respectively, and normalize later on, and Vitamin D was low 18.87 nmol/l (NR 75- 250). Urine culture (catheter sample) revealed E.Coli> 10,0000/mm³, sensitive to gentamycin and ampicillin. Electrocardiography and echocardiography were normal. Magnetic resonance imaging (MRI) of the brain on day-of-life 7 showed left basal ganglia and corona radiata subacute infarction extending to the posterior rim of the external capsule, and lateral part of the mid brain (Figure 1). Magnetic resonance angiography (MRA) of the brain and neck showed no evidence of arterial dissection, stenosis or hypoplastic branches. No treatment was given for the perinatal arterial stroke. The patient was discharged after 2 weeks of antibiotic therapy with therapy. The neurologic examination at discharge showed mild motor asymmetry with mild preferential deviation of the eyes to the left was noted. At 3 months of age, phenobarbitone was discontinued with no recurrence of seizure. At 9 months of age his weakness improved significantly, and he catch up with his motor, and language developmental milestone.
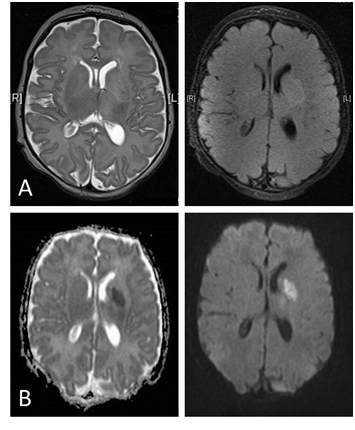
Figure 1 Magnetic resonance imaging of the brain.
(A) Axial T2 and fluid - attenuated inversion recovery ( FLAIR ) sequences showed a focal high- signal-intensity lesion in the body of the left caudate nucleus, putamen and left corona radiate.
(B) Diffusion - weighted image (DWI) sequences showed a high signal intensity at the left caudate nucleus, putamen and left corona radiate, the posterior rim of the external capsule extending down to the mid brain likely representing wallerian degeneration on diffusion sequences and low signalintensity on the apparent diffusion coefficient (ADC) map indicating Left basal ganglia and corona radiata subacute infarction.
The clinical scenario and the MRI findings of this neonate were consistent with perinatal stroke of arterial ischemic type.5 PAIS is a common cause of acute neonatal encephalopathy, and may manifest as seizures, altered mental status, hypotonia, sensorimotor deficits, and/or may manifest with subtle features like lethargy, feeding difficulties, temperature or hemodynamic instability. Our neonate presented with focalclonic seizures involving the contralateral limb (right arm), consistent with early symptomatic PAIS( 60% ), as the most common presenting symptom is neonatal seizures and of which 70-83% are focal seizures in the first 3 days of life.6-8 The presence of hypocalcemia 2nd to low vitamin D may also contribute to the clinical convulsion. There was no hypotonia or apnea which were reported in previous studies7,8 accounting for (41.9%) and (25.8%), respectively. Although he had reduced movement of right arm initially, there was remarkable improvement on discharge examination, and on later follow-up. Emerging hemiparesis with early handedness or hemiplegic cerebral palsy are the presenting sign in 25 % - 40% after the early months of life , at a median age of 8 months,5,7 while developmental delay, specific cognitive deficiency or seizures are noted in 40% of neonates.3
Cranial magnetic resonance imaging (MRI), especially with diffusion-weighting, is the most sensitive imaging modality for detecting PAIS, dating the injury, predicting the motor outcome of the child. Although ultrasonography with Doppler imaging of cerebral blood flow is useful in the neonate who is too ill to transport.4,9 Our neonate transfontanell US was normal, consistent with previous reported low sensitivity of US in PAIS especially in small, and deep seated infarction.10 MRI showed left basal ganglia and corona radiata subacute infarction observed in the distribution of the middle cerebral artery consistent with the literatures most reported PAIS in full term were left-sided, and involve the left middle cerebral artery (MCA) territory,2,9 with classical late thalamic atrophy.11 This predominance of lesions on the left side is thought to be due to differences in vulnerability, maturation or to the presence of vascular asymmetries. The left hemisphere may also be more vulnerable to embolic lesions either from a patent ductus arteriosus or from the left common carotid artery.12
Perinatal stroke is thought to have a diverse underlying aetiology and several potential risk factors in term infant are frequently cited.12-14 These include hereditary or acquired thrombophilias and environmental factors that occur before, during, and after delivery (Table 1). However, establishing a causal role for many risk factors awaits larger, more definitive, prospective or case-controlled studies.15
Maternal factors |
Pre-eclampsia |
Diabetes Mellitus |
Placental factors |
Placental infection |
Feto- maternal hemorrhage |
Fetal factors |
IUGR |
PROM |
Table 1 Risk factors Associated with perinatal Arterial Ischemic Stroke
IUGR: Intrauterine Growth Retardation; CS: Cesarean Section; HD: Heart Diseases; PDA: Patent Ductus Arteriosus; PA: Pulmonary Atresia; IEM: Inborn Errors of Metabolism; HC: Homocystinuria; OA: Organic Acidemias; CDG: Congenital disorders of glycosylation
*are the major thrombophilic abnormalities associated with PAIS
Our neonate had the following risk factors:
Gestational diabetes:Perinatal stroke in infant of diabetic mother is not uncommon. A recent retrospective study identified gestational diabetes as a major risk factor (8/11) followed by delivery complications especially hypoxia, and fetal distress,9 and in another case - control study it accounts for 16.1% versus 4.2%; P0.04 ,while maternal diabetes account for 3.4% among 32 neonates with PAIS.7 Curry et al.13 reported 3 mothers with gestational diabetes and 2 with pre-existing insulin-dependent diabetes among 60 cases of PAIS. In the 1st two studies instrumental delivery, and meconium stained liquor, respectively are the major risk factors. Several hypotheses may explain the mechanism of PAIS in gestational diabetes: Polycythemia that lead to hyperviscosity which increase the like hood of thrombosis, fetal size (large for gestational age) a condition favoring a difficult traumatic birth hypothesis with subsequent a asphyxia or cervico-cerebral arteries injuries, fetal distress with subsequent hypoxia. Another possible mechanism is a congenital malformations of cranial blood vessels ie., hypoplasia or congenital stenosis, or congenital heart defects , although this is more likely in infant born to mother with pregestational diabetes mellitus . Stenerson MB et al.16 reported a bilateral basal ganglia infarction in infant of diabetic mother during diabetic ketoacidosis.16 He proposed two possible mechanism 2nd to maternal ketosis. One that ketosis will cause uteroplacental insufficiency with subsequent reduced blood flow to deeper gray matter nuclei such as the BG and the thalami. Two, transplacental transfer of maternal ß- hydroxybutarate (ß-OHB ) during the episode of ketoacidosis can led to accumulation of ß-OHB in the fetus which will reduce fetal PaO2 and increase fetal lactate levels, thus causing decreased glucose fuel uptake by the fetal brain with subsequent infarction of the basal ganglia. In our patient, maternal ketosis by measurement of serum B- OHB was not determined. There was no evidence of polycythemia , traumatic delivery or asphyxia.
Systemic neonatal infection (urinary tract infection): It has been reported that central nervous system (CNS) infection or systemic infection may lead to hypercoagulable state, and can potentially increase the risk for thrombosis and cerebral ischemia by several pathogenic pathways.2
All newborns with septicemia are at great risk for cerebral infarct. Our newborn showed an evidence of infection clinically and on urine culture. No leukocytosis, or fever noted, but there was dehydration. Cerebrospinal fluid, and blood culture were negative. Concordance was observed in 13% of blood cultures and 3% of CSF cultures in neonate with positive urine culture.21 Negative blood culture can be contributed to poor blood culture sensitivity in neonate. Our neonate results showed low activity level of Protein C, and Protein S initially which can be explained by the presence of systemic infection. This was repeated at 6 weeks, and at 3 months post presentation, and the results were normal. The presence of two risk factors, may support the hypothesis that PAIS could be a multifactorial disorder Only neonatal supportive care, and phenobarbitoine were given for this neonate as in most cases antithrombotic therapy is not indicated for perinatal thromboembolic stroke which is usually a self-limited condition with low risk of progression or recurrence, and the possible side effects of anticoagulant drugs may be life-threatening3 exceptions according to the American College of Chest Physicians recommendation include patients at risk for recurrent arterial ischemic stroke due to congenital heart disease or an abnormal prothrombotic workup. Although PAIS has a low mortality,3 but it is a common cause of long - term neurologic disability. Neurodevelopmental outcome could not precisely be determined in our patient because of short term follow- up.
Gestational diabetes and systemic infectionscan predispose neonate to perinatal arterial ischemic stroke. Screening for PAIS in neonate with convulsion using magnetic resonance imaging (MRI) is crucial, especially if initial Transfontanelle ultrasound is normal or non-informative and the patient has potential risk factors for PAIS. Vulnerability of BG, and thalami to metabolic changes in gestational diabetes can be determined by further reports and studies.
This work was supported by the College of Medicine Research Center, Deanship of Scientific Research, King Saud University.
None.

©2016 Kentab. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.