Journal of
eISSN: 2373-6410


Case Report Volume 10 Issue 3
Correspondence: Dr. Bipin chaurasia, Chief Resident, Department of Neurosurgery, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
Received: March 06, 2020 | Published: May 28, 2020
Citation: Ahmed N, Alam S, Islam KMT, et al. Subependymal giant cell astrocytoma associated with hyperproteinorrhachia, presented with vp shunt malfunction: a rare clinical entity. J Neurol Stroke. 2020;10(3):97-99. DOI: 10.15406/jnsk.2020.10.00418
Subependymal giant cell astrocytomas (SEGAs) are WHO grade I tumors, most frequently associated with tuberous sclerosis complex (TSC). TSC sometimes also called Bourneville’s disease is a neurocutaneous disorder characterized by hamartoma of many organs like skin, brain, eye and kidneys. In this report, we illustrate the case of SEGA with hydrocephalus, who presented with features of raised intracranial pressure, along with shunt malfunction due to shunt valve blockage with proteinaceous material. Patient underwent interhemispheric transcallosal approach and complete removal of tumor followed by ventriculo- atrial shunt and achieved favourable outcome.
Tuberous sclerosis complex (TSC) is a neurocutaneous disorder, in which patient may present neurologically with features of subcortical tubers, subependymal nodules, and subependymal giant cell astrocytomas (SEGAs) (Table A). In rare cases, SEGA patients may demonstrate abnormally high levels of cerebrospinal fluid (CSF) protein (hyperproteinorrhachia), which is responsible for obstruction of CSF diversion devices in shunted cases. However, this phenomenon is rarely reported in the previous literature (Table 1). Here, we illustrate the long term beneficial effect of gross total removal of tumor in a symptomatic TSC patient with hyperproteinorrhachia without necessity of everolimus therapy.
|
Serial no. |
Author |
Year of publication |
Age (years)/sex |
Diagnosis |
Treatment |
|
1 |
Perek-Polnik et al.4 |
2012 |
10 / female |
SEGA with hydrocephalus |
External CSF drainage, followed by everolimus therapy |
|
2 |
Laviv et a.3 |
2015 |
21/male |
SEGA with hydrocephalus |
Repeated surgery, shunt revision, endoscopic fenestration followed by everolimus therapy |
|
2015 |
24/female |
SEGA with hydrocephalus |
Repeated surgery, shunt revision followed by everolimus therapy |
||
|
3 |
Kasper et al.5 |
2017 |
18/female |
SEGA with hydrocephalus |
VP shunt placement followed by surgery |
Table 1 Reported cases of SEGA with Hyperproteinorrhachia, leads to shunt malfunction
Diagnostic criteria of tuberous sclerosis complex16
A 12 years old male patient presented with insidious onset of dull aching headache and vomiting for one year, progressive dimness of vision in both eyes for the same duration (Figure 1). He experienced one episode of generalized tonic clonic convulsion followed by loss of consciousness. After evaluation with MRI of brain, he diagnosed as a case of SEGA with obstructive hydrocephalus and underwent urgent VP shunt surgery in a private hospital (Figure 2). However, 7 months following shunt surgery, patient admitted for definitive surgery into BSMMU with additional features of ascites. However, his liver function test, renal function test and cardiac evaluation were normal. Serum albumin, total protein and albumin- globulin ratio was also within normal limit. At this stage, we exteriorize the lower end of VP shunt and drain 1 litre ascitic fluid for decompression. We sent both CSF and ascitic fluid for biochemical, cytological and microbiological examination. Findings were normal except high protein level both in CSF and ascitic fluid. Later on, we came into conclusion that, CSF high protein level was responsible for impaired peritoneal absorption of CSF. Patient underwent anterior interhemispheric transcallosal approach and gross total removal of tumor. At 10th POD, he underwent right sided ventriculo-atrial shunt. His postoperative period was uneventful. 1 year after surgery, repeat MRI of brain with contrast showed no recurrence of tumor with well functioning status of the shunt (Figure 3).
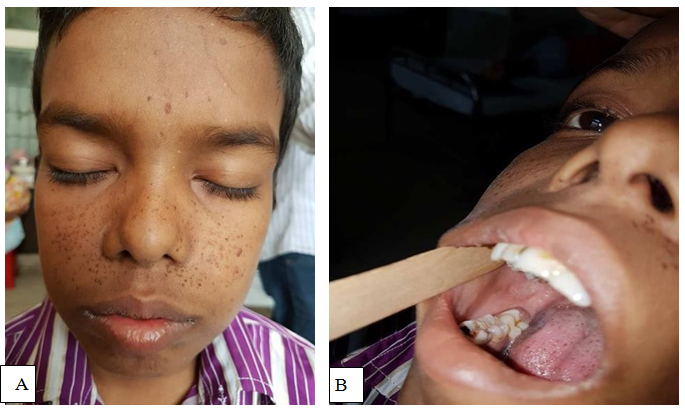
Figure 1 Photograph of the patient showing- adenoma sebaceum (A); non traumatic gingival fibroma (B).
According to the International TSC Consensus Conference in 2012, it was recommended that patient with SEGA associated with ventriculomegaly should undergo early surgical intervention for better long term outcome. The association of these tumors with a highly proteinaceous CSF has been mentioned as a reason to avoid shunt placement as the initial treatment in symptomatic SEGA patients.2,3 According to the previously published literature, there are several possible mechanism for hyperproteinorrhachia in CSF of a SEGA patient, such as presence of high levels of clotting proteins, recurrent hemorrhages into the tumor with spillage of the blood products into the CSF and meningeal spreading of the solid tumor.4–7 These explanations are less likely in our case, since there was no evidence of hemorrhage and the CSF cytology report was normal.
We have reviewed this rare observation, which were summarised in Table 1. We have hypothesized that the high protein content may have resulted from increased proteinaceous secretions by the tumor. However, further study required to prove the hypothesis.
Surgical resection of a SEGA tumor can not only relieve local mass effect and reverse blockage of the foramen of Monro; it can also reverse the grossly elevated CSF protein levels and prevent shunt malfunction. However, pathophysiology of this unique condition related to CSF malabsorption needs further evaluation for treatment modalities.
None.
The author declares no conflict of interest.

©2020 Ahmed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.