Journal of
eISSN: 2373-6410


Mini Review Volume 1 Issue 2
Department of Dementia and Higher Brain Function, Tokyo Metropolitan Institute of Medical Science, Japan
Correspondence: Fuyuki Kametani, Department of Dementia and Higher Brain Function, Tokyo Metropolitan Institute of Medical Science, Tokyo 156-856, Japan, Tel +81-3-6834-2349
Received: May 27, 2014 | Published: May 30, 2014
Citation: Kametani F. S100A9/Mrp14 plays an important role in Ab amyloidosis enhancement. J Neurol Stroke. 2014;1(2):31-33. DOI: 10.15406/jnsk.2014.01.00006
S100A9/Mrp14 plays a prominent role in the regulation of inflammatory processes and immune response. Recent findings suggest that S100A9/Mrp14 is involving Ab amyloidosis in cerebral amyloid angiopathy and Alzheimer's disease brain. In this review, I introduce what kind of role S100A9/Mrp14 has played in Ab amyloidosis.
Keywords: S100A9/Mrp14; Amyloid; Amyloidosis; Alzheimer's disease; Cerebral amyloid angiopathy; Inflammation; Aβ
AD, alzheimer's disease; CAA, cerebral amyloid angiopathy; APP–CTFs, C–terminal fragments of amyloid precursor protein
Recently, we reported the histopathological changes in cerebrovascular Aβ amyloid deposition between biopsied and autopsied findings in a Cerebral Amyloid Angiopathy (CAA) patient actively treated with corticosteroid, showing possible post–treatment regression of cerebrovascular Aβ amyloid deposits.1 To confirm the reduction of amyloid deposits and to clarify this mechanism, we analyzed amyloid and its related proteins from CAA with or without corticosteroid treatment using the proteomic techniques and compared their proteins' profiles.2 We found that vast amounts of S100A9/Mrp14 were associated with Aβ amyloid from CAA case without corticosteroid treatment and that S100A9/Mrp14 was significantly reduced in Aβ amyloid from the CAA extracts with corticosteroid treatment.2
S100A9/Mrp14 is a calcium– and zinc–binding protein which plays a prominent role in the regulation of inflammatory processes and immune response, and preferentially exists as a heterodimer or heterotetramer with S100A8 known as calprotectin (see Uniprot database, http,//www.uniprot.org/uniprot/P06702). S100A9/Mrp14 known to play an important role in the biological responses to vascular injuries by promoting leukocyte recruitment and in the damaged vessels with deposition of Aβ amyloid fibrils and this protein is supposed to regulate vascular inflammation.3 Moreover, a significantly increased microglia expression of S100A9/Mrp14 was observed in the temporal cortex of both familial and sporadic Alzheimer's disease (AD) cases.4 Therefore, S100A9 is a neuroinflammatory marker of AD.
Recently, it has been reported that S100A9/Mrp14 has the ability of amyloid fibril formation.5–8 Moreover, S100A9/Mrp14 interacts with the Aβ peptide and accelerates Aβ amyloid fibril formation.9–11 Furthermore, S100A9/Mrp14 drives microglia into a proinflammatory state thereby compromising microglial key function such as phagocytosis.12,13 Accumulation of Aβ amyloid fibrils on cerebral vessels and in brain parenchyma secondarily induces local inflammation.14 In the interactions of microglia/monocyte with Aβ, microglia has two complementary non–redundant roles, beneficial promotion of phagocytosis of Aβ and harmful production of neurotoxins and proinflammatory molecules.12,15,16 Proinflammatory molecule, S100A9/Mrp14 is secreted by activated phagocytes and strongly up– regulated in numerous inflammatory condition.17 An elevated level of S100A9/Mrp14 on Aβ amyloid fibril deposits induces further inflammation around amyloid fibril deposits, enhances Aβ amyloid firbrils formation and additionally drives microglia into a proinflammatory state thereby compromising microglial phagocytosis.12,13 BACE1 activity is also elevated in this condition, resulting in increased Aβ production.18 Increased levels of S100A9/Mrp14 are further accelerated by this inflammation. This vicious cycle conceivably expands the deposition of Aβ amyloid fibrils including amyloid–associated proteins on cerebral vessels and in brain parenchyma as shown in Figure 1.
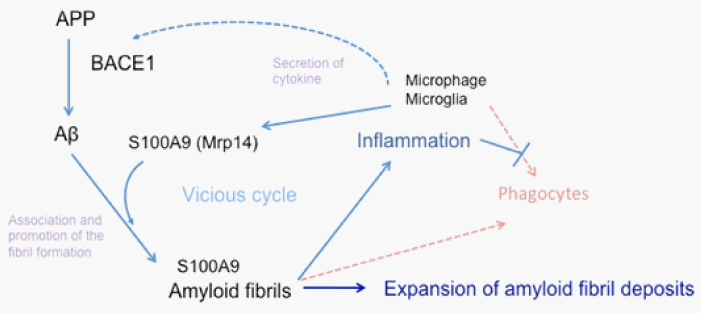
Figure 1 A comprehensive model of amyloid fibril formation enhancement. Local inflammation by Aβ amyloid deposition induces S100A9 production by activated phagocytes and up– regulation of the inflammatory condition.
Thereafter, S100A9 induces increased formation of Aβ amyloid fibril and further inflammation. Moreover, S100A9 drives microglia into a proinflammatory state thereby compromising microglial phagocytosis.
Interestingly, in APP/PS1 transgenic mice, S100A9/Mrp14 was found to be unregulated in microglial cells surrounding amyloid plaques and in APP/PS1–transgenic mice deficient for S100/Mrp14, a decrease of key cytokines involved in APP processing, a reduction of BACE1 expression and activity and a reduction of overall Aβ deposition were observed.12 In Tg2576 transgenic mice, knockdown by short hairpin RNA or knockout of the S100A9/Mrp14 gene significantly reduced the neuropathology, greatly improved the learning and memory impairment and reduced the amount of Aβ and APP–CTFs by increasing neprilysin and decreasing BACE1 activity.19,20 Furthermore, S100A9 knockdown mice displayed an increased spatial reference memory in the Morris water maze task and Y–maze task as well as decreased Aβ neuropathology because of reduced levels of Aβ, C–terminal fragments of amyloid precursor protein (APP–CT) and phosphorylated tau.21 These suggest that S100A9/Mrp14 may be a key protein of AD pathology.
Inhibition of Aβ production and/or promotion of Aβ clearance are regarded as valuable strategy in AD therapy. So far, almost all clinical trials against Aβ amyloid including γ−secretase and β–secretase (BACE1) did not get the expected results. Therefore, the inhibition or the decrease of S100A9/Mrp14 is a possible target for Aβ amyloidosis. In CAA and in AD, corticosteroid treatment suppresses the secondarily induced vascular inflammation1,14,20,22 and the activation of S100A9/Mrp1.2,23 Reduction of S100A9/Mrp14 may induce suppression of amyloid fibril formation9 and increase phagocytosis of fibrillar Aβ in microglia cells,12 resulting in reduced Aβ amyloid fibril deposition. Deposited Aβ amyloid fibrils seem to be gradually metabolized by the inherent elimination processes including an action of microglia and perivascular macrophages.24–26 Corticosteroid treatment is considered to be a potentially effective treatment for Aβ amyloidosis in AD and CAA.1,22,27
S100A9/Mrp14 activates inflammatory condition on cerebral vessels and in brain parenchyma, enhances Aβ amyloid firbrils formation, and drives microglia into a proinflammatory state thereby compromising phagocytosis. S100A9/Mrp14 may be a key protein of AD pathology and is a possible target for Aβ amyloidosis in brain.
None.
The authors declare there are no conflicts of interest related to the article.

©2014 Kametani. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.