Journal of
eISSN: 2373-6410


Research Article Volume 9 Issue 5
1Russian Medical Academy for Continuing Professional Education, Moscow, Russia
2Pichugin City Clinical Hospital for Children, Perm, Russia
3Novokuznetsk Chied Clinical Psychoneurological Sanatorium, Novokuznetsk, Russia
4Regional Child Hospital, Tomsk, Russia
5Regional Child Clinical Hospital, Belgorod, Russia
6Evpatoria City Child Clinical Hospital,Yevpatoriya, Russia
7City Clinical General Hospital No. 4, Diagnostic and Consulting Center for Children, Ivanovo, Russia
8Vologda Child City Polyclinic No. 3,Vologda, Russia
9Lipetsk City Child Hospital, Lipetsk, Russia
Correspondence: VP Zykov, Russian Medical Academy for Continuing Professional Education, Moscow, Russia
Received: June 06, 2019 | Published: October 28, 2019
Citation: Zykov VP, Serebrennikova EB, Panchenko TN, et al. Results of a multicenter study on the efficacy of cortexin in treatment of cognitive dysfunction in children. J Neurol Stroke. 2019;9(5):300-304. DOI: 10.15406/jnsk.2019.09.00393
To study the efficacy and tolerability of cortexin in the treatment of cognitive dysfunction in children.
Material and methods: The study included 635 patients, aged 3–7 years. Patients were divided into 4 clinical groups: group 1 (269 children with attention deficit hyperactivity disorder (ADHD)), group 2 (215 children with speech delay), group 3 (82 patients with the consequences of a perinatal lesion of the central nervous system), group 4 (69 patients with asthenic/neurotic syndrome). Attention, visual memory and thinking were assessed before and after treatment. Standard treatment of cortexin, including 10 intramuscular injections, was used.
Results and conclusion: The reliable effect of cortexin on cognitive impairment was shown. The best response to treatment was observed in patients with ADHD, aged 3–4 years, in particular on the tests measuring thinking. The tolerability of cortexin was good. It has been concluded that cortexin is an effective drug for treatment of children with ADHD, speech delay and consequences of a perinatal lesion of the central nervous system.
Keywords: cortexin, attention deficit hyperactivity disorder, speech delay disorder, cognitive dysfunction
According to the results of one of the latest foreign researches,1 neurological and mental abnormality among children aged 6–10 years reaches 18%, herewith attention deficit hyperactivity disorder (ADHD) heading the list is observed in 5.8% of patients, speech pathology - in 3.42%, learning disability - in 3.26%, anxiety and depressive disorders - in 2.4% and behavioural disorder - in 1.8%, at the same time half of children have comorbid symptoms. It is authors’ opinion that the majority of such children need additional training classes and social assistance.
In the course of the screening neuropsychological examination of children and adolescents living in Moscow, it was found that 17% of them have school maladaptation that is evident as learning disability.2 Under considered pathological conditions along with pedagogic and psychotherapeutic care, it is reasonable to search and use up-to-date drugs which would not have any psychotropic effect, could stimulate brain plasticity and help to cope with cognitive and asthenic disorders. In this regard attention is drawn to cortexin.
Cortexin is a complex of neuropeptids of animal origin with a mechanism of brain plasticity stimulation. The following proteins interacting with cortexin were found in the brain: three neuron-specific β5-tubulins (components of cytoskeleton microtubules) and 14-3-3 α/β protein classified as an adapter protein influencing other peptides; actin participating in neurons migration, reparation and differentiation (cytoskeleton protein existing in many tissues); B-type creatine kinase is the enzyme of cell energy exchange.3 During experimental studies, the ability of the drug to modulate dopamine content in the brain4 was shown. Cortexin comes from the brain tissues of beeves. The quality of material, including its safety in terms of possible prion disease, is carefully controlled. The technique of Cortexin production excludes any possibility of prion contamination. Cortexin is sold in a form of lyophilizate. Since the drug has polypeptide nature, it is impossible to conduct properly pharmacokinetics studies.
Cortexin use in pediatrics started in the 2000s. Most of the studies5–8 were dedicated to the therapy of motor and cognitive impairments where therapeutic effect was noted. Cortexin contributed to the restoration of psychomotor skills and correction of attention-deficit and hyperactivity signs in patients with ADHD.7,9 According to the data obtained from conducted clinical trials and more than 20 years of pharmacovigilance practice was registered the following possible adverse events: anaphylaxis, hypersensitivity to components of the drug, redness at the injection site, anxiety, rise of the body temperature, asthenia, shivers, psychomotor agitation, dystaxia, headache, dizziness, sleepiness, hyperesthesia, tachycardia, arrhythmia, insomnia, high blood pressure, angioedema, erythema, hives, rash, itches, allergodermia. Any of these possible adverse events are very rare (<1/10 000).
Further studying of сortexin therapeutic benefit in child neurological practice is required. In particular, assessment of its efficacy in cases of cognitive dysfunctions secondary to ADHD, developmental speech delay (DSD), consequences of central nervous system perinatal lesion (perinatal CNS lesion consequences), asthenoneurotic syndrome (ANS) is of the utmost interest and is the purpose of this study.
635 patients aged 3-7 years divided into four groups were involved into the study: The 1st group included 269 children with ADHD, the 2nd group 215 children with DSD, the 3rd group 82 patients with perinatal CNS lesion consequences, and the 4th group consisted of 69 children with ANS characterised with emotional instability, undue fatiguability, headaches and sleep disturbances. All observation groups were stratified by age. One age period was 3-4 years, the other was 5-7 years.
Psychological assessment of cognitive functions was performed in the form of tests adapted to children’s age, and took 30-35 minutes during the patient examination.10 In this regard, all the children were divided into two age groups: the 1st subgroup included 142 children being 3-4 years old, the 2nd subgroup included 492 patients aged 5-7 years.
Cortexin was used in the form of a standard course consisting of 10 intramuscular injections. Herewith, different types of solvents were studied: 1) water for injection (in 32.3% of patients); 2) physiological solution (in 15.9%); 3) 0.5% novocaine solution for pain treatment (in 51.8% of patients). IM injections are the only one route of administration, registered nowadays. Since the drug has polypeptide nature, it cannot be administered per os. IM route of administration was initially selected for drug development since it is an easy and effective way to administer Cortexin. Some other possible routs of administration are currently under discovery. Cortexin is administered once daily, preferably at the same time of the day. An administration course of 10 injections showed its efficacy when studied during drug development program.
Patients were examined during two visits. During the 1st visit, before treatment, the state of attention, thinking and visual memory was assessed. Then the patients received a treatment consisting of 10 injections at a dose of 10 mg for children weighing over 20 kg and 0.5 mg for children weighing less than 20 kg. During the 2nd visit, tests for cognitive functions were repeated.
Test results were statistically processed using Statistica 12 program (StatSoft Inc., USA). The differences were compared using Wilcoxon test, Pearson’s chi-square, and Mann-Whitney tests; the differences of the compared parameters at the level of р<0.05 were defined as tests of significance. The acceptability of the treatment was also evaluated quantitatively by the following criteria: excellent (5 points) no side effects and deviations in laboratory test values; good (4 points) short-term mild side effects or insignificant deviations in laboratory test values which do not require treatment correction; satisfactory (3 points) moderate side effects or significant deviations in laboratory test values which require treatment correction; bad (2 points) moderate to severe side effects or significant deviations in laboratory test values which require drug discontinuation. Doctors’ satisfaction with the treatment efficacy and acceptability was also assessed.
The research was conducted according to the approved protocol, the recommendations of the International conference on harmonization (ICH GCP), the principles of the Helsinki Declaration and provisions of the national standard of the Russian Federation about good clinical practice (GCP) of GOST P 52379-2005. The research was approved by independent committee on ethical examination of clinical trials 18.12.2015. Each patient/legal representative/guardian has signed the informed consent to participate in the study. The study involved 8 research centers. Dates of the study: September 2016 - April 2017.
All patients included in a research completed the period of active treatment and observation according to the protocol of a research. No patient dropped out of the study ahead of schedule. The Table 1 below represents high efficacy in patients of various ages in all cognitive spheres (attention, memory, and thinking). As it is shown, the drug effect was not age-dependant.
|
Test |
Visit |
Average±Average Standard Deviation |
Median |
Minimum |
Maximum |
p (Wilcoxon Test) |
|
Patients aged 3–4 years |
||||||
|
Attention |
1st |
4.07±0.21 |
3.00 |
0.00 |
13.00 |
<0.001 |
|
|
2nd |
6.13±0.24 |
6.00 |
0.00 |
16.00 |
|
|
Thinking |
1st |
3.74±0.15 |
4.00 |
0.00 |
9.00 |
<0.001 |
|
|
2nd |
5.59±0.18 |
6.00 |
1.00 |
10.00 |
|
|
Visual memory |
1st |
3.77±0.21 |
4.00 |
0.00 |
10.00 |
<0.001 |
|
2nd |
5.75±0.27 |
6.00 |
0.00 |
14.00 |
|
|
|
Patients aged 5–7 years |
||||||
|
Attention |
1st |
7.44±0.15 |
7.00 |
0.00 |
17.00 |
<0.001 |
|
|
2nd |
10.57±0.16 |
11.00 |
1.00 |
17.00 |
|
|
Thinking |
1st |
5.60±0.08 |
5.00 |
0.00 |
10.00 |
<0.001 |
|
|
2nd |
7.45±0.08 |
7.00 |
1.00 |
10.00 |
|
|
Visual memory |
1st |
5.95±0.14 |
5.00 |
0.00 |
14.00 |
<0.001 |
|
2nd |
8.61±0.14 |
8.00 |
0.00 |
14.00 |
|
|
Table 1 Assessment of the cognitive functions status before and after cortexin treatment (points)
In the course of the study, no undesirable side reactions were detected against the background of Cortexin's use in the recommended treatment plan. Drug tolerability was defined as excellent and it was identical in both age groups; also no differences in treatment acceptability depending on the solvent type were found, the expectation of an injection is apparently more significant for a child, than the intervention itself.
As for the results of cortexin treatment in separate clinical groups, they are given in Figure 1. Significant changes in results for attention, visual memory and thinking tests were found in ADHD group by the 2nd visit (Table 1). Earlier L.S. Chutko9 gave similar data on cortexin high efficacy for patients with ADHD with the predominance of attention disorder. This may be explained with the ability of the drug to modulate dopamine neurotransmitter system function and with the stimulation of the child’s developing brain plasticity.3,4 Similar data were also obtained in other clinical groups (Figure 1), which may be explained with the syndrome interinfluence mechanism in the system of neuronal-functional brain networks.
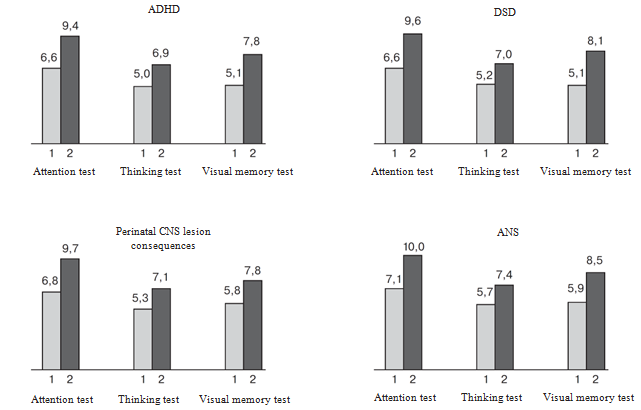
Figure 1 Test Results for Separate Psychological Functions in Groups of Patients with ADHD, DSD, Perinatal CNS Lesion Consequences, and ANS. Average Values of the Studied Parameters (points).
1 and 2 are visits. In all the cases, the differences between the 1st and the 2nd visits are significant (p<0.001).
Increase of attentional function potentiates speech function, visual memory, and thinking. In this regard, it is reasonable to note the following regularity: maximum significant differences in thinking tests were determined in the group at infant age limit that may be a “therapeutic window” for cortexin in patients with mental development disorder.
Our data on speech function improvement after cortexin treatment correlate with the conclusions of I.I. Ogorodova et al.11 As an example, we attach one of our observations. Patient P., 6 years old, has pronunciation disorder and poor attention.
According to the medical history: The child has burdened perinatal history: pregnancy in the setting of gestational toxicosis. Timely delivery. Birth weight: 3820 g, body length: 51 cm.
Apgar score: 7/8 points. Is observed by a neurologist from the 1st month with the following diagnosis: perinatal CNS lesion consequences, movement disorders syndrome. Received outdoor treatment (nootropics, vasoactive agents, massage, physiotherapy). Motor development was normal, but developmental speech delay was observed (phrase speech only since 3 years old). Encephabol treatment received from January 2017.
Neurological status: General satisfactory state. Normal head shape. Clear consciousness.
Behaviour without any abnormalities: well balanced, contacts willingly. Social skills are developed: the child dresses himself, gets undressed with assistance, eats with no outside help; tidiness skills are developed. Memory is weakened.
Cranial nerves (CN): I CN-olfaction is retained; II CN-optic nerve function is not disordered; III, IV, VI CN-full range of eyeball movements; no strabismus, pupils of normal size; no anisocoria; direct and consensual pupillary light reflex. No ptosis and nystagmus are present. V CN-trigeminal nerve exit points are unpainful; corneal reflexes are retained. VII CN-symmetrical face; eye fissure S=D; no lacrimation or eye mucosa dryness. VIII CN-hearing is not impaired. IX, X, XII CN-voice is loud; swallowing is not disordered; tongue is in midline; pharyngeal reflex is retained. XI CN-torticollis is absent.
Sensitive function: not disordered.
Motor function: active range of motion is not restricted. Muscular tone is decreased in proximal parts of hands, back muscles, legs-satisfactory (D=S); feet are flat.
Biceps and triceps reflexes, carporadial reflex (D=S), knee-jerk (D=S) and ankle-jerk (D=S) reflexes, abdominal upper, middle, lower reflexes are brisk and symmetrical. Pathological reflexes: Babinski’s and Gordon’s symptoms, Oppenheim’s syndrome, palmomental reflex-are absent. Manner of walking is correct. Maintains stable posture in Romberg’s position, correctly performs coordination tests; no hyperkinesis.
Speech: phrasal, agrammatical, dearticulation. Vocabulary is insufficient: counts to 10 automatically, knows primary colours and tones, geometrical figures, “right-left”, distinguishes fruits from vegetables, knows seasons, but confuses signs, generalizes, distinguishes the odd one out.
On the basis of the clinical study, the following diagnosis was determined: developmental articulation disorder. Assigned therapy: logopedic therapy; massage of speech apparatus; 10 intramuscular cortexin injections.
Repeated inspection after one month.
Neurological status: clear consciousness, willingly answers questions, meaning of questions is clear. Attention concentration is improved. The number of wrong pronounced sounds is reduced, vocabulary is extended. Positive dynamics is detected-improvement of speech function.
In addition to the data given above, positive cortexin influence on the results of intergroup comparison with the assessment of correct answers gain may be confirmed. They are given in Figure 2.
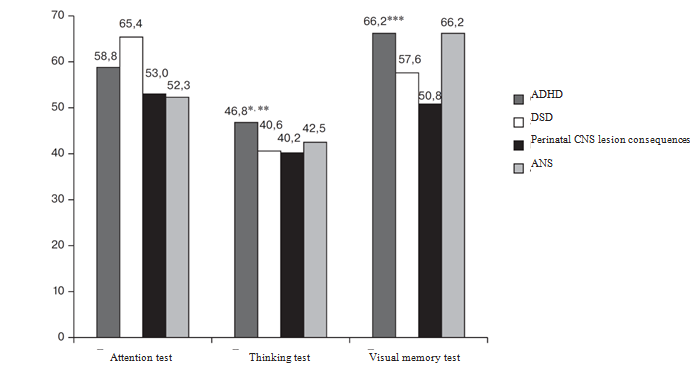
Figure 2 Average Gain of Correct Answers among Examined Children (in %).
* — difference between ADHD and DSD groups (p=0.006); ** — difference between ADHD and ANS groups (p=0.007); *** — difference between ADHD and perinatal CNS lesion consequences groups (p=0.019).
Significant differences were found between the indicators of the 1st (ADHD) and 2nd (DSD) group patients in thinking tests (p<0.006), which presupposes that patients of the 2nd group have developmental disorder of impressive speech, which is characterised by task understanding problems and the correct answers number decreasing respectively. It is necessary to clarify significant differences in thinking tests in the ADHD group and the 4th (ANS) group, which include patients with psychoemotional disorders, since there is a possibility that correct answers number decrease is connected with the children’s anxiety before another task.
On the basis of the performed cortexin efficacy study among children aged from 3 to 7 years, its positive effect in cases of ADHD, DSD, perinatal CNS lesion consequences, and ANS was manifested. Multimodal cortexin action was confirmed, which may be explained with the influence of drug peptides on the brain dopaminergic system and brain plasticity.
A kind of therapeutic window determined at the age of 3–4 years presupposes the possibility of active drug use (up to several courses per year) in children of infant and preschool age.
It is reasonable to continue the studies in groups with developmental speech disorder with the division of patients into subgroups with impressive speech (sensory dysphasia) and expressive speech (motor dysphasia) disorders.
None.
The authors declare no conflicts of interest.
None.

©2019 Zykov, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.