Journal of
eISSN: 2373-6410


Case Report Volume 14 Issue 1
1Department of Neurology, Mohamed V Military Teaching Hospital, Morocco
2Department of Neurophysiology, Mohamed V Military Teaching Hospital, Morocco
Correspondence: Yassine El-Adraoui, Department of Neurology, Mohamed V Military Teaching Hospital, Mohamed V University, Rabat, Morocco, Tel +212 634 693 795
Received: January 22, 2024 | Published: March 6, 2024
Citation: El-Adraoui Y, Hamid M, Alloussi H, et al. Progressive myelopathy revealing primary Sjogren syndrome: a case report. J Neurol Stroke. 2024;14(1):17-19. DOI: 10.15406/jnsk.2024.14.00574
Background: Central nervous system (CNS) involvement in primary Sjogren Syndrome (pSS) is infrequent and often poses a diagnostic dilemma due to its diverse clinical presentation.
Case presentation: A 66-year-old woman presented to our department with a three-month history of progressive weakness in the lower limbs, sensory loss, and sphincter dysfunction. Spine MRI revealed an intramedullary hyperintense signal on T2 weighted images extending longitudinally and transversally from D6 to D12. Auto-immune tests, viral serologies, and metabolic workups yielded negative results. A minor salivary gland biopsy confirmed chronic grade III sialadenitis. Primary Sjogren Syndrome was diagnosed based on the 2016 ACR-EULAR classification criteria. The patient underwent treatment with glucocorticoids and cyclophosphamide, showing mild improvement after six months.
Conclusion: This case highlights the significance of considering Sjogren syndrome in the differential diagnosis of progressive myelopathy. Given the severity of spinal involvement in pSS, early diagnosis and aggressive treatment from the disease’s onset are crucial.
Keywords: progressive myelopathy, central nervous system, sicca symptoms, primary sjögren syndrome, cyclophosphamide
Primary Sjögren syndrome (PSS) is an autoimmune exocrinopathy characterized by the association of xerophthalmia, xerostomia, and systemic manifestations of an immuno-inflammatory nature.1 Central nervous system (CNS) involvement is relatively rare compared to peripheral nervous system (PNS) involvement in SS. Large-scale studies have reported CNS involvement in 2 to 5% of cases.2,3 Sjögren syndrome may affect various regions of the CNS, including the spinal cord, optic nerves, brainstem, cerebellum, and cerebral hemispheres.4 In this report, we present a rare case of progressive myelopathy as the initial manifestation of Primary Sjogren syndrome.
A 66-year-old female patient was admitted to the Neurology department with a three-month history of progressive weakness in the lower limbs, sensory loss, and sphincter dysfunction. She had a known history of diabetes and hypertension. There was no family history of systemic disease. She reported a year-long history of knee arthralgia treated as gonarthrosis and experienced eye dryness.
Upon admission, neurological examination revealed spastic paraplegia with an MRC (Medical Research Council Scale for Muscle Strength) score of 0/5 in both lower limbs. Muscle stretch reflexes were brisk in the patella and ankle, with no Babinski sign present. Upper limbs were unaffected. Light touch, pinprick, temperature, and position sense were impaired in the trunk and lower limbs circumferentially, with a sensory level at D6. She also exhibited sphincter disorders, including urinary and bowel incontinence. Additionally, cranial nerves were intact, and there was no cognitive impairment.
A whole spine MRI (Magnetic Resonance Imaging) revealed an intramedullary hyperintense signal on T2-weighted images extending longitudinally from D6 to D12 and transversally in axial planes. These findings indicated longitudinally extensive transverse myelitis (Figure 1 & 2). T1-weighted images following Gadolinium injection showed mild enhancement.
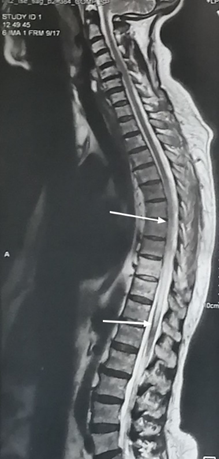
Figure 1 Sagittal T2-weighted whole spine MRI showing intramedullary hyperintensity extensive longitudinally from D6 to D12 (1a).
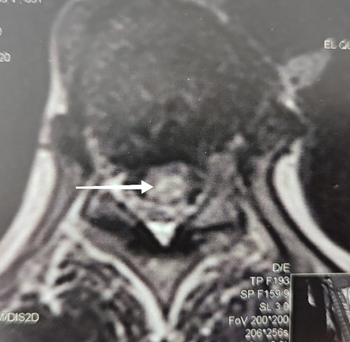
Figure 2 Axial T2 weighted image (D11 level) showing intramedullary hyperintensity with transverse extension.
Cerebral spinal fluid (CSF) analysis found normal cell count and proteinorachia at 0.45 g/l, with no intrathecal synthesis of IgG. Blood count, biochemistry, thyroid hormones, protein, vitamin B12, and folic acid levels were all within normal ranges. Serology tests for HIV, HBV, HCV, Syphilis, and Borrelia were negative. Autoimmunity tests for Antinuclear antibodies (ANA), Anti-DNA, Anti-neutrophil cytoplasmic antibodies (ANCA), Anti-Ro/SSA, Anti-La/SSB, Anti-Aquaporin-4 (Anti-AQP4) and Anti-Myelin Oligodendrocyte Glycoprotein (Anti-MOG) were negative. Brain MRI showed vascular leukopathy patterns with no specific hyperintensities of white matter, while thoraco-abdominopelvic computed tomography was normal.
A minor salivary glands biopsy confirmed chronic grade III sialadenitis based on the Chisholm and Masson score. Ophthalmic examination revealed an ocular staining score > 5, along with a positive Schirmer’s test. An exclusion assessment, including thyroid peroxidase antibodies, angiotensin conversion enzyme, immunoglobulins weight assay, and serum complement C3 and C4, yielded normal results. PSS was diagnosed according to the ACR/EULAR 2016 criteria.
The patient received high dose intravenous Methylprednisolone for five days, followed by oral prednisone (60mg/day) for four weeks, with a gradual tapering dose down to a minimum of 10 mg/day. Cyclophosphamide was administered at a dose of 1g/month for six months, followed by a dose of 1g every three months. The patient also underwent physiotherapy that was continued after discharge. At the six-month follow-up, a mild improvement was observed in the muscular strength of the lower limbs. The left limb’s muscular strength increased from 0/5 to 2/5 on the MRC scale, while the right limb demonstrated an increase from 0/5 to 1/5.
The diagnosis of PSS is based on the 2016 ACR-EULAR classification criteria.5 Classification of Sjogren’s syndrome is determined by summing up the weights of the following items, resulting in a score of ≥4 (Table 1): labial salivary gland biopsy with focal lymphocytic sialadenitis and focus score ≥1 (3), positive anti-SSA(Ro) antibodies (3), ocular staining score ≥5 on at least one eye (1), Schirmer test ≤5 mm/5 min on at least one eye (1), and unstimulated whole saliva flow rate ≤0.1 ml/min (1). In our patient, the ACR sum score was calculated as 5. Importantly, she did not exhibit any conditions listed as exclusion criteria, notably no history of head and neck radiation treatment, active hepatitis C infection, acquired immunodeficiency syndrome, sarcoidosis, amyloidosis, graft vs. host disease, or IgG4-related diseases.
Item |
Weight / Score |
Labial salivary gland with focal lymphocytic sialadenitis and focus score ≥ 1. |
3 |
Anti-SSA (Ro) + |
3 |
Ocular staining score ≥ 5 (or van Bijsterfeld score ≥4) on at least one eye |
1 |
Schirmer ≤5 mm/5min on at least one eye 1 |
1 |
Unstimulated whole saliva flow rate ≤0.1 ml/min |
1 |
Table 1 ACR-EULAR Classification criteria for primary Sjögren’s syndrome (pSS)5
Neurological manifestations of PSS can occur as the initial manifestation or present later in the course of the disease.6 They predominantly affect the PNS (10%) and less commonly the CNS (2 to 5%), with focal or multifocal involvement.7 Spinal involvement in PSS may manifest as acute or subacute transverse myelitis, rarely as progressive myelitis with episodes of relapse and remission. Progressive forms of myelitis are associated with sensory symptoms, urinary incontinence, and gait disturbances that can progress to spastic paraplegia,8 as observed in our patient. Optic neuritis and transverse myelitis can be seen in patients with PSS,9 either isolated or overlapping with neuromyelitis optica spectrum disorder (NMOSD).10 Recent reports highlight the association between NMOSD with positive anti-AQP4 antibodies and Sjogren’s syndrome.11,12 Additionally, cases of co-occurrence of Sjogren's syndrome and multiple sclerosis have also been described.13 Other neurological manifestations of Sjogren’s syndrome include focal or generalized seizures, cognitive dysfunction, cerebellar ataxia, encephalopathy, and motor neuron disease-like syndrome.7,9
The pathogenic mechanisms of CNS involvement in PSS are not yet fully understood. It has been suggested that the main pathogenetic mechanism may be related to immunologically mediated small vessel vasculopathy leading to ischemia.14 Other possible mechanisms include direct infiltration of the CNS by mononuclear cells or vascular involvement related to antineuronal antibodies. The heterogeneity of CNS involvement in PSS and our incomplete knowledge of its pathogenic mechanisms make it challenging to propose a standardized therapeutic regimen. However, the accepted therapeutic approach involves intravenous methylprednisolone for 3-5 days in the acute phase, followed by oral prednisone and cyclophosphamide in monthly boluses.15 Cyclophosphamide has shown particular efficacy in cases of myelopathy, with improvement or stabilization of disability, although the data primarily come from small series or case reports.16
Other immunosuppressants, such as azathioprine, cyclosporine, and rituximab can be considered as well.17 Plasmapheresis and immunoglobulin IV may be considered in resistant cases. More recently, subcutaneous tocilizumab, an IL-6 receptor inhibitor, was successfully used to treat a patient with progressive Sjogren myelopathy refractory to cyclophosphamide.18
CNS involvement in primary Sjogren Syndrome can manifest rarely as progressive myelopathy, commonly presenting with sensory symptoms, sphincter disorders, gait disturbance, and potential progression to spastic paraplegia. This case underscores the importance of considering Sjogren’s Syndrome in the differential diagnosis of progressive myelopathy. Clinicians should screen carefully for sicca symptoms and other indications of rheumatologic disease, proceeding with a lip minor salivary gland biopsy if needed. Early diagnosis and proactive treatment from disease onset are crucial for the course and prognosis of CNS involvement in PSS.
The authors have no conflicts of interest to declare.
None.
Informed consent for publication was obtained from the patient.

©2024 El-Adraoui, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.