Journal of
eISSN: 2373-6410


Research Article Volume 13 Issue 3
1MD, Neurosurgeon, Hospital Municipal Salgado Filho (HMSF/ RJ), Neurosurgery Service, Brazil
2MD, Doctoral Student in Neuroscience, Institute of Biomedical Sciences (ICB), Universidade Federal do Rio de Janeiro (UFRJ), Brazil
3MD, PhD, Oncologist, Emergency Course Supervision. Hospital Municipal Salgado Filho (HMSF/RJ), Brazil
4MD, PhD, Cardiologist, Emergency Course Coordinator. Hospital Municipal Salgado Filho (HMSF/RJ), Brazil
5MD, Neurosurgeon, Emergency Course Preceptor, Hospital Municipal Salgado Filho (HMSF/RJ), Brazil
6MD, Neurosurgeon-in-Chief, Hospital Municipal Salgado Filho (HMSF/RJ), Brazi
Correspondence: Francisco Eduardo Penna Doutel de Andrade, MD, Neurosurgeon, Salgado Filho Municipal Hospital (HMSF/RJ), Neurosurgery Service, Rio de Janeiro, RJ, Brazil
Received: July 05, 2023 | Published: July 18, 2023
Citation: Andrade FEPD, Leser FS, Souza H, et al. Primary intracerebral hematoma (PICH): arterial hypertensive neurosurgical emergency. J Neurol Stroke. 2023;13(3):60-70. DOI: 10.15406/jnsk.2023.13.00547
Introduction: PICH is the acute and organized intracerebral bleeding, resulting from primary causes, especially systemic arterial hypertension. It is a common entity with very high morbidity and mortality. However, its surgical indications are controversial.
Method: Observational, prospective study with 532 patients with PICH and hypertension seen between April 2002 and 2016.
Aim: To analyze clinical and tomographic parameters for classifying patients with PICH between candidates for conservative or surgical treatment.
Results: The main clinical findings were motor deficit and obnubilation. The most prevalent topography was lobar and 40% were associated with hemoventricle. 55% were operated on and craniotomy and external ventricular shunt were the most performed. The predictors of surgical treatment were lobar topography, presence of hemoventricle and hematoma volume between 31-50 ml. A lethality of 49% was observed with a tendency to decrease among those who underwent surgery. This benefit was greater in lobar PICH between 31-50ml and with thalamic or nuclear lesion associated with hemoventricle.
Conclusion: The study identified the main parameters associated with the indication of surgical treatment and positive outcome in patients with PICH. Although further studies are needed, it is suggested to consider surgery in thalamic or nuclear PICH with 31-50ml associated with hemoventricular hydrocephalus and lobar PICH with 31-50ml and Glasgow Coma and Pupillary Scale (GCS-P) > 3.
Keywords: intracerebral hemorrhage, systemic arterial hypertension
Systemic arterial hypertension (SAH) is present in 20-30% of adults in developed countries and is even more common in population subgroups such as men, blacks and the elderly.1–3 Of these patients, an important portion evolves during the course of the disease with complications considered hypertensive emergencies, among which the primary intracerebral hematoma (PCIH) is one of the most important.1–6
ICH is defined as acute and organized intracerebral bleeding. This can be of primary etiology (ICHP), associated mainly with severe and/or uncontrolled SAH, or secondary, when resulting from causes such as intracranial aneurysms, cerebral vascular malformations, trauma, apoplectic tumors and infections.7,8 It is a condition of emergency importance, given its high morbidity and mortality, as well as the hypertension itself, which besides being serious, is a condition of good prognosis if adequately treated.2–4,6–9
PICH has an incidence of 13 to 15 cases/100,000 inhabitants/year, with a lethality rate of 35 to 50% in the first month after ictus, and is the cause of 3,700 to 5,400 deaths/year in the USA.9–13 Clinical factors such as age, use of oral anticoagulants, presence of comorbidities such as previous heart disease and concomitant diseases such as respiratory infections; the clinical picture at the time of admission, such as lowered consciousness; as well as factors related to the hematoma, such as the presence of ventricular extension and its increased volume, stand out as important factors for the prognosis of these patients with ICHP.7,9,14–26
Although it is a potentially lethal condition of great relevance in the context of neurosurgical emergencies, and of quick and easy diagnosis in most emergency departments in the country equipped with neuroimaging exams,25 the management of PICH remains a widely controversial topic in the medical literature. It is known that the adequate selection of patients candidates to surgical approach is one of the most important prognostic factors and, although these prognostic factors are widely studied, there is still no unanimous criterion for the indication of surgery in these patients8,10,11,27–42
In this context, the present study aims to analyze the factors that contribute to the clinical and tomographic gradation of HICP, in order to establish reliable parameters that allow the most immediate classification of patients with installed HICP, with the intention of favoring the triage decision by the emergency assistant whether the treatment will be conservative or surgical.
This is a single-center longitudinal, observational, prospective study based on the analysis of 532 patients with ICH and SAH seen at the emergency unit of Hospital Municipal Salgado Filho (HMSF) from April 2002 to April 2016. Exclusion criteria were the presence of subacute or chronic conditions with an evolution time > 24 hours until hospital admission and the presence of factors that could classify patients as having secondary intracerebral hematoma, such as trauma, aneurysms, arteriovenous malformations, infections and tumors. Patients with hematomas in other extracerebral encephalic regions, such as the cerebellum and/or the brainstem, were not considered in the present study, by the very definition of the nomenclature and/or topography of the object of study.
The patients underwent a complete clinical-neurological examination and CT scan upon admission and 14 days later, and the clinical variables considered in the study were the presence of headache, focal motor deficit and the Glasgow Coma and Pupil Scale (GCS-P), the radiological variables of the topography of the hematoma, - subclassified as lobar, nuclear, thalamic or ventricular, - and the volume of the hematoma, measured by the Method of Kothari et al, further classified as proposed by Kramer et al in 2005,12 into hematomas of volume less than or equal to 30 ml, from 31 to 50 ml and greater than 50 ml; presence or absence of brain atrophy or perilesional edema. These additional variables considered, atrophy or edema, respectively increase or decrease intracranial volumetric compliance, interfering with the level of consciousness, the deeper the topography.10–12 While atrophy is a pre-PICH condition, edema is observed more after 24h of installed HIPC,12 excluding edema as the object of the present study, delimited until 24h of installation.
Statistical calculations were performed using the rstatix library in R language through the RStudio software. To verify association between categorical variables, such as hematoma volume, clinical picture, topography and patient outcome, Chi-square test (X2) of independence (Pearson) was used with residuals adjusted by Bonferroni post-hoc test to identify exact values that differ from the expected count. A statistical significance level of 95% was assumed (p<0.05; adjusted residual > 1.96 or < -1.96).
As a statistical base strategy for sample calculation, a pilot data collection between 1998 and 2000 was used (data not included in the final analysis of this study) to estimate the incidence of negative outcome (lethality) among patients of the unit undergoing or not undergoing surgery. With these data, we used a sample size calculation formula to estimate the difference between two incidences considering 95% confidence (Zα/2 =1.96) and tolerable absolute error of 5% and a two-sided test formula to compare two proportions (incidences) with statistical power of 90% (Zβ =1.645).
This study was performed by the team from the Neurosurgery Service of the Hospital Municipal Salgado Filho, in the city of Rio de Janeiro, in collaboration with the Postgraduate Program in Morphological Sciences of the Institute of Biomedical Sciences of the Federal University of Rio de Janeiro. The project was reviewed and approved by the Medical Ethics Committee of Hospital Municipal Salgado Filho. All study procedures are in accordance with the Helsinki Declaration for research involving human patients.
We analyzed 532 cases of PICH in patients with SAH admitted to the Emergency Department of HMSF from April 2002 to April 2016. The study sample had approximately equal gender distribution, with 51% (n=272) being female and the remaining 49% (n=261) being male. The most prevalent age groups were between 51 and 60 years old with 33% of cases (n=175), as well as between 61 and 70 years old with 35% of cases (n=186). Most patients analyzed were known to have hypertension, 69% of the total number of cases (n=367) (Table 1).
|
n |
% |
||
|
Sex |
Male |
261 |
49.06% |
|
Female |
272 |
51.13% |
|
|
Age (years) |
≤ 30 |
6 |
1.13% |
|
31-40 |
34 |
6.39% |
|
|
41-50 |
86 |
16.17% |
|
|
51-60 |
175 |
32.89% |
|
|
61-70 |
186 |
34.96% |
|
|
≥ 71 |
45 |
8.46% |
|
|
Prior SAH |
Yes |
367 |
68.98% |
|
No |
112 |
21.05% |
|
|
Ignored |
53 |
9.96% |
|
|
Total |
532 |
100% |
Table 1 Descriptive analysis of demographic data of the sample of patients included in the study. The distribution between genders, age groups and occurrence of previously reported SAH were taken into consideration. Values of % refer to the total of patients (column)
As for the clinical-neurological picture, the main findings were Motor deficit in 46% of patients (n=245), lowered level of consciousness in 38% (n=201), 17% with GCS-P between 09-12 (n=92), 18% with GCS-P 04-08 (n=93) and 3% with GCS-P ≤ 3 (n = 16). Regarding the distribution of cases according to the topography of the PICH we observed that they had hemoventricular 40% of patients (n=212), lobar lesions 34% patients (n=183), lesions in deep base nucleus topography 14% of patients (n=75) and thalamic hematomas 12% of patients (n=62). Regarding the volume of the lesions, on the other hand, the most observed range, in 49% of patients (n=263), was ≤ 30ml, followed by lesions between 31-50ml, in 25% of cases (n=148) and finally lesions ≥ 51ml in 23% of cases (n=121) (Table 2). Respective examples in Figure 1.
|
|
|
n |
% |
|
Topography |
Ventricular |
212 |
39.85% |
|
Lobar |
183 |
34.40% |
|
|
Base Cores |
75 |
14.10% |
|
|
Thalamic |
62 |
11.65% |
|
|
Volume |
≤30ml |
263 |
49.44% |
|
31-50ml |
148 |
27.82% |
|
|
≥51ml |
121 |
22.74% |
|
|
Clinical Picture |
Motor deficit |
245 |
46.05% |
|
RNC |
201 |
37.78% |
|
|
GCS-P 09 to 12 |
92 |
17.29% |
|
|
GCS-P 04 to 08 |
93 |
17.48% |
|
|
GCS-P ≤ 03 |
16 |
3.01% |
|
|
Headache |
86 |
16.17% |
|
|
Total |
|
532 |
100.00% |
Table 2 Absolute number (n) and percentage (%) of patients with HIPC by clinical picture, topography and volume of the lesion. Values of % with respect to the total column (total patients)
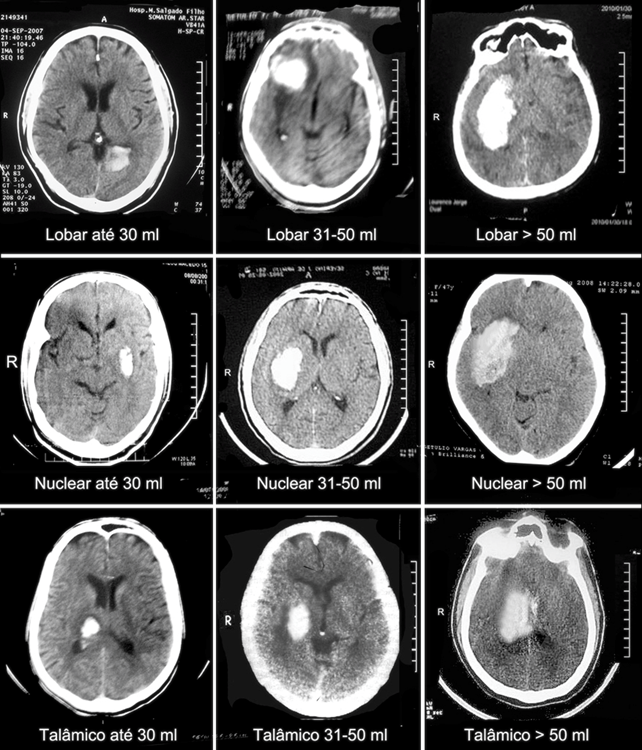
Figure 1 Examples of PICH extracted from the case series of this article, classified by skull CT, as to topography and volume of the lesion, except for ventricular lesions.
When the hemoventricular patients (n=212) were analyzed in isolation, the origin of the ventricular extravasation of the parenchymal bleed was mostly from thalamic lesions (59%, n=124), and in smaller proportions were nuclear and lobar lesions with 20% and 21% of the cases respectively (n=46 and n=48) (Table 2).
In this sense, we could evaluate the significant association between topography of the initial lesion and the risk of complication with hemoventricle occurrence (X2 = 96.96; p<0.0001). Approximately 67% of thalamic lesions evolved with ventricular extravasation, a higher risk when compared with only 37% and 19% of nuclear and lobar lesions respectively (Table 3).
|
Hemoventricle |
|||||||||
|
|
|
Present |
Absent |
|
|
||||
|
|
|
n |
% |
Adjusted residuals |
n |
% |
Adjusted residuals |
X² |
p |
|
Topography |
Lobar |
43 |
19.03% |
-8.431 |
183 |
80.97% |
8.431 |
96.96 |
< 0.001 |
|
Nuclear |
45 |
37.50% |
-0.597 |
75 |
62.50% |
0.597 |
|||
|
Talamic |
124 |
66.67% |
9.263 |
62 |
33.33% |
-9.263 |
|||
|
Volume |
≤30ml |
118 |
44.87% |
2.337 |
145 |
55.13% |
-2.337 |
7.51 |
0.023 |
|
31-50ml |
46 |
31.08% |
-2.565 |
102 |
68.92% |
2.565 |
|||
|
≥51ml |
48 |
39.67% |
-0.046 |
73 |
60.33% |
0.046 |
|||
|
Total |
|
212 |
39.85% |
|
320 |
60.15% |
|
|
|
Table 3 Distribution and bivariate statistics of patients with hemoventricle, according to topography and volume of the lesion. Value of p refers to the result of the test of X2. Values of % in relation to the total of the lines
We then analyzed the clinical presentation of patients associated with lesion topography and volume, respectively (Figures 2A & 2B). Smaller lesions course mostly with headache and motor deficit (24%, n=64 and 56%, n=147). Intermediate sized lesions, between 31 and 50ml, have a higher proportion with decreased level of consciousness, more specifically with GCS-P 09-12 (36%, n=53). The larger lesions, on the other hand, course with moderate to severe lowering with GCS-P 04-08 and GCS-P ≤3 (35%, n=42 and 10%, n=12 respectively) (Table 4).
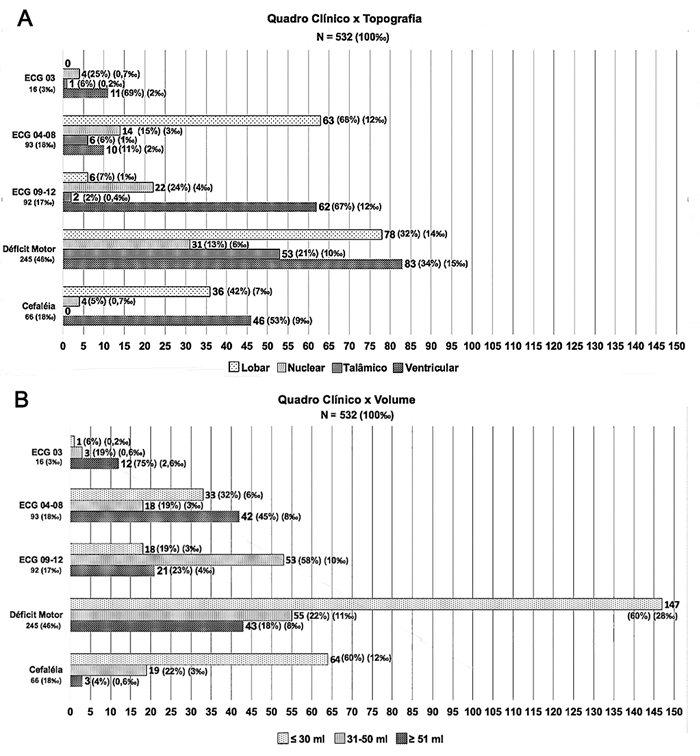
Figure 2 (A) Distribution and relative frequency of cases according to clinical picture and topography of HICP. (B) Distribution and relative frequency of cases according to clinical picture and lesion volume.
|
|
|
Headache |
Motor deficit |
GCS-P 09-12 |
GCS-P 04-08 |
GCS-P = 03 |
|
|
|
|||||||||||||||||
|
|
|
Total |
n |
% |
RA |
n |
% |
RA |
n |
% |
RA |
n |
% |
RA |
n |
% |
RA |
X² |
p |
|||||||
|
Volume |
≤ 30 ml |
263 |
64 |
24,33% |
5,061 |
147 |
55,89% |
4,503 |
18 |
6,84% |
-6,301 |
33 |
12,55% |
-2,963 |
1 |
0,38% |
-3,508 |
135,14 |
< 0,001 |
|||||||
|
31-50 ml |
148 |
19 |
12,84% |
-1,294 |
55 |
37,16% |
-2,554 |
53 |
35,81% |
7,011 |
18 |
12,16% |
-2,005 |
3 |
2,03% |
-0,822 |
||||||||||
|
≥ 51 ml |
121 |
3 |
2,48% |
-4,653 |
43 |
35,54% |
-2,640 |
21 |
17,36% |
0,021 |
42 |
34,71% |
5,677 |
12 |
9,92% |
5,063 |
||||||||||
|
Topography |
Lobar |
183 |
36 |
19,67% |
1,591 |
78 |
42,62% |
-1,149 |
6 |
3,28% |
-6,189 |
63 |
34,43% |
7,452 |
0 |
0,00% |
-2,941 |
159,15 |
< 0,001 |
|||||||
|
Nuclear |
75 |
4 |
5,33% |
-2,749 |
31 |
41,33% |
-0,885 |
22 |
29,33% |
2,975 |
14 |
18,67% |
0,292 |
4 |
5,33% |
1,272 |
||||||||||
|
Talamic |
62 |
0 |
0,00% |
-3,679 |
53 |
85,48% |
6,627 |
2 |
3,23% |
-3,116 |
6 |
9,68% |
-1,721 |
1 |
1,61% |
-0,684 |
||||||||||
|
Ventricular |
212 |
46 |
21,70% |
2,821 |
83 |
39,15% |
-2,600 |
62 |
29,25% |
5,933 |
10 |
4,72% |
-6,309 |
11 |
5,19% |
2,398 |
||||||||||
Table 4 Bivariate statistical analysis of the clinical picture manifested by patients with PICH by lesion topography and volume. p value refers to the result of Pearson's Chi-square test. RA corresponds to the values of adjusted residuals of the Chi-square test for each condition. Values of % with respect to the total of the lines
We also observed a close association between clinical picture and topographic pattern. In the sample of this study, patients with lobar lesions presented significantly more than the others with GCS-P 04-08 (34%, n=63), although motor deficit was also quite frequent, while nuclear and ventricular lesions presented more moderate degrees of decreased level of consciousness, especially with GCS-P 09-12 (29%, n=22 and 29%, n=62 respectively). Thalamic lesions, on the other hand, mostly presented with some degree of motor deficit (85%, n=53) (Figure 2A & 2C).
The treatment employed was surgical in 55% of the patients (n=292), and the others were treated conservatively (n=240) (Figure 3A). Regarding the operative techniques used in these patients, the one of choice in most cases was craniotomy, performed in 52% of the patients submitted to surgery (n=153), followed by External Ventricular Bypass (EVD) in 33% of the patients (n=97), Craniectomy in 9% (n=26) and trepanopuncture in 6% (n=16) (Figure 3B).
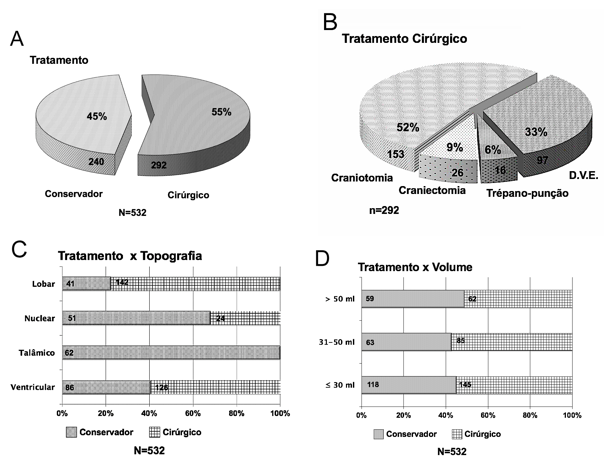
Figure 3 (A) Distribution of cases by therapeutic option. (B) Distribution according to the operative technique. (C) Treatment modality by PICH topography. Number of cases in the bars. (D) Distribution of cases according to treatment modality in relation to PICH volume. Number of cases in the bars (total N = 532).
A significant association was also observed between the topography of the primary lesion and the treatment modality employed (X2 = 131.81; p<0.0001) (Table 4). Surgical treatment was preferentially performed in patients with lobar lesions, of which 78% of patients underwent surgery (n=142), as well as in lesions with ventricular extension, which were operated on in 59% of cases (n=126). No patients with thalamic PICH and only 32% of patients with nuclear lesions (n=24) underwent surgical treatment (Figure 4C & Table 5).
|
|
|
|
Conservative |
Surgical |
|
|
||||
|
|
|
Total |
n |
% |
Adjusted Residuals |
n |
% |
Adjusted Residuals |
X² |
P |
|
Topography |
Ventricular |
212 |
86 |
40.57% |
-1.715 |
126 |
59.43% |
1.715 |
131.18 |
< 0.001 |
|
Lobar |
183 |
41 |
22.40% |
-7.622 |
142 |
77.60% |
7.622 |
|||
|
Nuclear |
75 |
51 |
68.00% |
4.298 |
24 |
32.00% |
-4.298 |
|||
|
Talamic |
62 |
62 |
100.00% |
9.240 |
0 |
0.00% |
-9.240 |
|||
|
Volume |
≤30ml |
263 |
118 |
44.87% |
-0.113 |
145 |
55.13% |
0.113 |
1.04 |
0.593 |
|
31-50ml |
148 |
63 |
42.57% |
-0.732 |
85 |
57.43% |
0.732 |
|||
|
≥51ml |
121 |
59 |
48.76% |
0.917 |
62 |
51.24% |
-0.917 |
|||
|
Total |
532 |
240 |
45.11% |
|
292 |
54.89% |
|
|
|
|
Table 5 Bivariate statistical analysis of the treatment modality applied to patients with PICH by lesion topography and volume. Value of p refers to the result of the Chi-square test. Values of % with respect to the total of the lines
As for the volume of the HICP, it was observed that this factor alone was not determinant for defining the surgical management (X2 = 1.094; p=0.593) (Table 5). Surgical treatment was performed in 51% (n=62) of the cases of lesions with volume greater than 50ml, as well as in 57% (n=85) and 55% (n=145) of the cases of patients with lesions with volume between 31-50ml and less than or equal to 30ml, respectively (Figure 4D).
Also regarding the choice of therapy employed for each case, it was observed that the parameters volume, topography and association with hemoventricity were those most associated with the decision of surgical approach of the lesions. The deepest PICH (thalamic and nuclear) with volume between 31-50ml deserved surgery only when there was associated hydrocephalus, through the implantation of LVAD, while those with volumes less than 30ml did not benefit from surgery (Figure 4).
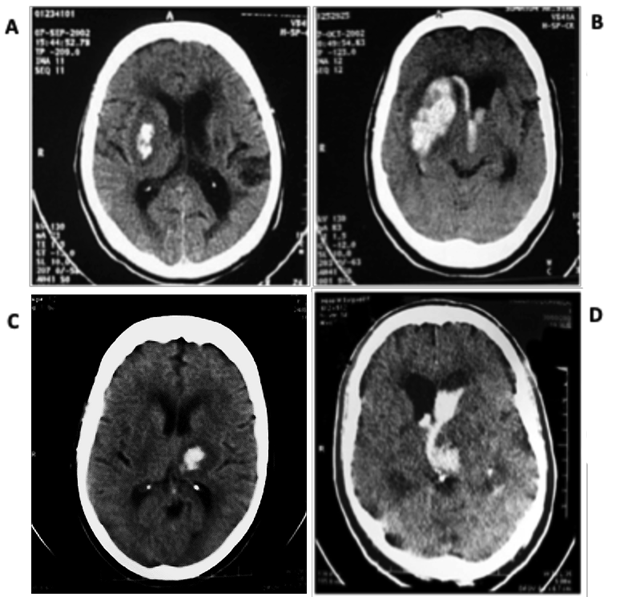
Figure 4 Images of the casuistry of the present study. (A) Nuclear IHCP on the right with volume of up to 30 ml and (B) Right nuclear IHCP with volume of 31 to 50 ml, with hydrocephalus caused by the arising hemoventricle, with mass effect and deviation of midline structures, indicating surgical drainage and LVAD. (C) Thalamic PICH with volume up to 30 ml, with halo of perimetral absorptive edema, without relevant mass effect, conservative treatment. (D) Thalamic IHCP with hemoventricle and hydrocephalus, with surgical indication (LVAD placement).
Lobar PICH with ≤30ml did not benefit from surgery, as did those with ≥ 51 ml, especially when associated with reduced level of consciousness, GCS-P ≤09 points. In lobar hematomas with volume from 31 to 50 ml, benefit by surgery (craniotomy) was observed (Figure 5), except in cases that presented with GCS-P ≤3 points.
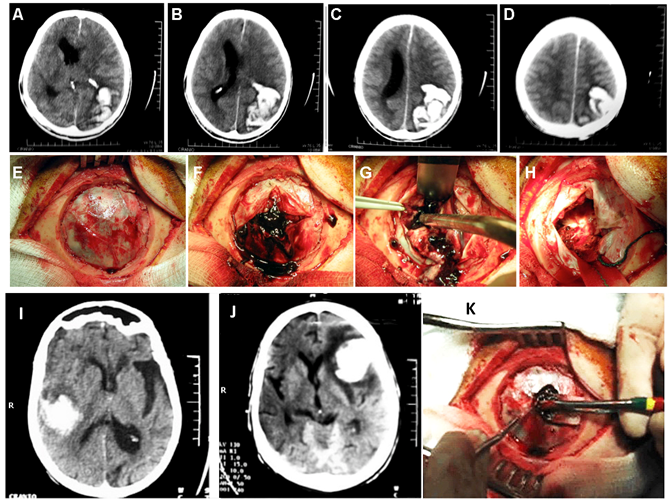
Figure 5 (A-D) Skull CT showing left parieto-occipital lobar PICH > 50 ml with marked mass effect and deviation of midline structures, with emergency surgical indication, as in E-H presented. (I) Right temporal lobar HICP, 31 to 50 ml with signs of associated atrophy, as noted enlargement of the left sylvian notch, without deviation of midline structures, without immediate surgical indication. J: Left frontal hematoma with a volume of 31 to 50 ml, with perilesional edema and significant mass effect, with marked contralateral deviation of midline structures, with surgical indication (craniotomy), as presented in K.
Regarding the overall outcome of patients, at the end of the study period, 32% of patients died (n=169) (Figure 6 & Table 6). Although no statistical significance was found regarding this data, a trend towards a lower lethality value was observed among patients treated surgically when compared to those who underwent conservative treatment (28% x 36%, RR = 0.793, 95%CI 0.619-1.017, X2 = 3.003; p=0.083) (Figure 7A & 7B, Table 7A & 7B).
|
|
|
Conservative |
Surgical |
|
|
|||||
|
|
|
Total |
n |
% |
Adjusted residuals |
n |
% |
Adjusted residuals |
X² |
p |
|
Outcome |
High |
363 |
154 |
64.17% |
-1.826 |
209 |
71.58% |
1.826 |
3.003 |
0.083 |
|
Death |
169 |
86 |
35.83% |
1.826 |
83 |
28.42% |
-1.826 |
|||
|
Total |
532 |
240 |
45.11% |
|
292 |
54,89% |
|
|
|
|
Table 6 Bivariate statistical analysis of association between applied treatment and global outcome. p value refers to the result of the Chi-square test with Yates correction. Values of % in relation to the total of the respective rows
|
A |
||||||||||
|
Conservative |
||||||||||
|
|
|
High |
Death |
|
|
|||||
|
|
|
Total |
n |
% |
Adjusted residuals |
n |
% |
Adjusted residuals |
X² |
p |
|
Topography |
Ventricular |
86 |
69 |
80.23% |
3.879 |
17 |
19.77% |
-3.879 |
27.432 |
< 0.001 |
|
Lobar |
41 |
19 |
51.22% |
-2.614 |
22 |
53.66% |
2.614 |
|||
|
Nuclear |
51 |
38 |
72.55% |
1.736 |
13 |
25.49% |
-1.736 |
|||
|
Talamic |
62 |
28 |
48.39% |
-3.624 |
34 |
54.84% |
3.624 |
|||
|
Total |
|
240 |
154 |
64.17% |
- |
86 |
35.83% |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
B |
|
|
|
|
|
|
||||
|
Surgical |
||||||||||
|
|
|
|
High |
Death |
|
|
||||
|
|
|
Total |
n |
% |
Adjusted residuals |
n |
% |
Adjusted residuals |
X² |
p |
|
Topography |
Ventricular |
126 |
98 |
77.78% |
2.047 |
28 |
22.22% |
-2.047 |
10.29 |
0.006 |
|
Lobar |
142 |
100 |
70.42% |
-0.425 |
42 |
29.58% |
0.425 |
|||
|
Nuclear |
24 |
11 |
45.83% |
-2.918 |
13 |
54.17% |
2.918 |
|||
|
Talamic |
0 |
0 |
0.00% |
- |
0 |
0.00% |
- |
|||
|
Total |
|
292 |
209 |
71.58% |
|
83 |
28.42% |
|
|
|
Table 7 Bivariate statistical analysis of association between overall outcome and topography of lesions treated clinically (A) or surgically (B). Values of % with respect to the total of the respective rows
In the group of patients treated conservatively, the most incident topographies were thalamic lesions in 39% of cases (n=34) and lobar in 25% (n=22), followed by ventricular and nuclear in 20% and 15% respectively (n=17 and n=13) (Figure 7A).
Still regarding mortality, of the operated patients who died of ICHP, the most commonly identified topography of the lesion was lobar in 50% (n=42), followed by ventricular lesion in 34% of the cases (n=28) and finally lesions in deep base nuclei in 16% (n=13) (Figure 7B).
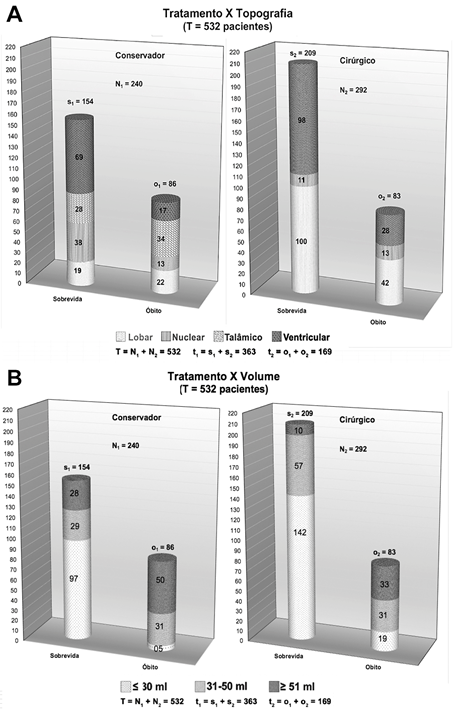
Figure 7 Distribution charts of global and case-specific evolution according to conservative or surgical treatment according to topography (A) and volume (B) of the HICP.
A significant association between lesion topography and both surgical (X2 = 10.29; p=0.006) and clinical (X2 = 27.43; p<0.001) mortality was also observed (Table 8A & 8B). As for the operated patients, nuclear lesions were associated with a significantly higher risk of death than ventricular lesions, with a lower risk of mortality (Table 8B). As for patients treated conservatively, thalamic and lobar lesions were positively associated with the occurrence of death, while ventricular lesions were also associated with a lower risk (Figure 8D). If the volume of the lesion was considered, it was also related to the primary outcome observed and to the benefit of surgical treatment. It was observed that there was no significant difference in overall survival between operated or non-operated patients with lesions up to 30ml or over 51ml (X2 = 2.800; p=0.0942 & X2 = 1.511; p=0.219, respectively). However, patients who presented with intermediate lesions, between 31 and 50ml, had a significantly lower relative risk of death when undergoing neurosurgical procedure (RR=0.746; 95% CI 0.540-0.996). Of these patients, only 48% (n=29) of those treated conservatively survived at the end of the study follow-up time, versus 64% (n=57) of those treated surgically (X2 =3.314; p=0.0466).
|
A |
||||||||||
|
Conservative |
||||||||||
|
|
|
High |
Death |
|
|
|||||
|
|
|
Total |
n |
% |
Adjusted residuals |
n |
% |
Adjusted residuals |
X² |
p |
|
Volume |
≤ 30 ml |
102 |
5 |
4,90% |
8,591 |
97 |
95,10% |
8,591 |
76.094 |
< 0.001 |
|
31-50 ml |
60 |
31 |
51,67% |
-2,953 |
29 |
48,33% |
2,953 |
|||
|
≥ 51 ml |
78 |
50 |
64,10% |
-6,337 |
28 |
35,90% |
6,337 |
|||
|
Total |
|
240 |
86 |
35,83% |
|
154 |
64,17% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
B |
|
|
|
|
|
|
|
|
|
|
|
Surgical |
||||||||||
|
|
|
High |
Death |
|
|
|||||
|
|
|
Total |
n |
% |
Adjusted residuals |
n |
% |
Adjusted residuals |
X² |
P |
|
Volume |
≤ 30 ml |
161 |
19 |
11,80% |
-6,982 |
142 |
88,20% |
6,982 |
73.216 |
< 0,001 |
|
31-50 ml |
88 |
31 |
35,23% |
1,693 |
57 |
64,77% |
-1,693 |
|||
|
≥ 51 ml |
43 |
33 |
76,74% |
7,607 |
10 |
23,26% |
-7,607 |
|||
|
Total |
|
292 |
83 |
28,42% |
|
209 |
71,58% |
|
|
|
Table 8 Bivariate statistical analysis of association between overall outcome and volume of lesions treated clinically (A) or surgically (B). Values of % with respect to the total of the respective rows
When performing the crossing of mortality, clinical or surgical, with volume, it must be considered the anatomical structures themselves, - nucleus base and thalamus - alone and together do not support volumes above 50 ml, it is understood why they were not operated when smaller than 31 ml and without hemoventricle and/or hydrocephalus (Figure 7 & Table 8, where we observe the global comparison between the evolution and the treatment used, conservative or surgical, considering the variables of topography and volume).
PICH is usually an acute complication of SAH, whose pathophysiology consists of the extrapolation of the tolerance limit of intracranial vessels to hypertensive peaks, causing bleeding and subsequent organization of hematoma in the intimacy of the brain parenchyma.5,14,43-45,56
In agreement with the literature, our data show the lethal potential of this condition, with 10 to 20 cases/100,000 inhabitants/year, constituting 10 to 20% of all strokes, being the third cause of death in industrialized countries.2,4,8,9,15,17-23,46–51,56 Other causal factors include chronic alcoholism and use of drugs (thrombolytics, antiaggregants, anticoagulants).
It affects individuals mainly from the 4th and 5th decades of life, with a predominance of males and blacks, as well as more occurring in the colder seasons of the year, following the general prevalence of SAH.6,10–13,18,30–33,38,42,56 The findings of the present study and previous ones from the same group, showed a reversal of gender prevalence, predominantly female. It is possible that this atypical epidemiological phenomenon is related to the well-known survival bias, since females tend to reach older ages and spontaneously seek health care more often. Another plausible explanation is the difference in the composition of the study population itself, with a majority of patients residing in the Northern Zone of the city of Rio de Janeiro.
Topographically, this type of lesion is closely related to vascular architecture, affecting perforating vessels and, less frequently, cortical branches of cerebral arteries.44,52,53 Through this concept, we understand why, as suggested by the results of this study, the most common locations of this type of lesion are lobar, thalamus, and the deep basal nuclei.
As a result of the primary bleeding, there may or may not be extravasation into the cerebral ventricular system, according to the vector of bleeding and the volume itself. Therefore, thalamic topography hematomas were the main cause of hemoventricle in the patients studied, analogously to what is observed in previous studies on this conduction, mainly due to the anatomical contiguity with the ventricles.9,23,24,53–55
The clinical-neurological picture was related to the volume and topography of the hematoma, as expected, considering the relationship between anatomy, pathophysiology and propaedeutics of brain lesions. We highlight the association between the predominance of motor deficit as the main clinical manifestation and the absence of hemoventricle, as well as with smaller volume lesions. On the other hand, consciousness disorders were more associated with lesions with the presence of hemoventricle, as well as with larger lesions, in agreement with previous studies and the pertinent literature.6,10–13,18,30–33,38,42
Likewise, it was found in the study an important association between the volume of the IHCP and its respective topography, being the largest volume hematomas more associated with lobar topography, while those of intermediate volume tended to be thalamic with hemoventricle or lobar, and the smallest hematomas tended to be thalamic without and with hemoventricle. This lower occurrence of nuclear and thalamic hematomas when considering larger volume lesions is explained by the reduced size of the anatomical compartments in question, which do not hold large volumes of blood mass, generally above 50 ml.6,8,25,34,44
According to the most current succession of evidence, the therapeutic strategies with well-defined benefit after establishment of PICH are:
Invasive monitoring of intracranial pressure (ICP) is a reasonable measure for patients with severe PICH and decreased level of consciousness and, if hydrocephalus is evident, ventricular drainage is the definitive measure, while therapy with hyperosmolar solution has an uncertain benefit.7,26
Regarding the neurosurgical approach to hematomas, there is strong evidence to support its indication. It is understood that patients with PICH between 20-30ml and moderate reduction of the level of consciousness tend to benefit from treatment by minimally invasive evacuation of the hematoma by stereotactic or endoscopic aspiration, with or without the use of thrombolytic.7,10,57 However, this method is still questionable, due to the nuclear or thalamic topography, with risks of worsening bleeding, by the method itself, without an adequate direct control of the involved structures, deep and with a rich microvascular network.58
The use of LVAD has been proven to reduce mortality in patients with hemoventricle and decreased level of consciousness, and the associated use of thrombolytics is also a promising strategy, although the benefit remains uncertain. Regarding craniotomy its evidence remains inconclusive even for patients with moderate to high severity ICHP. It is commonly used as a heroic measure for patients with progressive deterioration of clinical and neurological status.7,26
The factors that influenced the decision for surgical approach, as well as the therapeutic results of the present study, in view of previous studies and the literature consulted, were in agreement.6–8,20–23,27–42 Mainly the topography of the lesion, but also the clinical picture presented, were determinant for this selection and kept an important correlation with the surgical outcome. Regarding the volume of the HICP, this was not associated in isolation to the choice of therapy applied, but indirectly, through stratified subsequent analysis, if considered in conjunction with the topography, and both before the clinical picture.
Deep HICP, thalamic and nuclear, underwent surgery only with volume up to 30 ml when there was associated hydrocephalus, through deployment of LVAD to control secondary intracranial hypertension, while superficial lobar hematomas underwent an invasive approach to a greater extent. Lobar hematomas with volume above 50 ml showed benefit with conservative treatment when considering the overall outcome of mortality, while in the 31 to 50 ml range the surgical results and mortality of patients varied significantly with respect to the clinical status presented. This fact indicates the need to reconsider conservative treatment for this specific subgroup of patients.
Regarding the most relevant points of the study presented, especially noting the observational study design chosen to carry out the investigation, it allowed us the broad analysis and with a large population universe of multiple possible factors associated with the diagnosis, the overall outcome and the best therapeutic choice of patients with PICH in the p opulation studied, in order to assist in the identification of these patients, as well as in the process of rapid suspicion of the main candidates for surgical approach to this condition.59,60
However, it is also worth noting the main limitations of the observational method applied, which calls attention mainly to the need for further studies in order to better understand the clinical and pathophysiological importance of the parameters emphasized by this study, especially clinical picture, volume and topography of the PICH for the unfavorable evolution of patients, as well as to define their statistical weight through multivariate modeling and establish an index that serves in practical terms for the decision of the best therapeutic option and best prognostic gradation.
Hospital Municipal Salgado Filho (HMSF/RJ), Neurosurgery Service, Rio de Janeiro, RJ, Brazil. Post-Graduation Program in Morphological Sciences, Biomedical Sciences Institute, Federal University of Rio de Janeiro, RJ, Brazil.
Ethics Committee approval: approved by the Medical Ethics Committee, Hospital Municipal Salgado Filho (HMSF/RJ).
None.
The authors declare no conflicts of interest.
None.

©2023 Andrade, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.