Journal of
eISSN: 2373-6410


Case Report Volume 5 Issue 4
Istanbul university, Turkey
Correspondence: Nilufer Yesilot, Istanbul university, Millet Cad, Turkey
Received: October 07, 2016 | Published: December 13, 2016
Citation: Atmaca MM, Çoban O, Tuncay R, Yesilot N (2016) Patient with Systemic Lupus Erythematosus Presenting with Ischemic Stroke, Convexal Subarachnoid Hemorrhage and Subdural Hematoma at Different Times. J Neurol Stroke 5(4): 00185. DOI: DOI: 10.15406/jnsk.2016.05.00185
MRI,magnetic resonance imaging; CSF, cerebrospinal fluid; SLE, systemic lupus erythematosus; ASA, acetylsalicylic acid; CT, computed tomography; FLAIR, fluid attenuated inversion recovery; OACs , oral anticoagulants
A 17year old female presented with mild right hemiparesis and choreiform arm movements. She had malar rash and renal impairment. Antinuclear (ANA) and anti-DNA antibodies were positive. Cranial magnetic resonance imaging (MRI) showed bilateral periventricular white matter lesions. Cerebrospinal fluid (CSF) findings were normal. Systemic lupus erythematosus (SLE) and neurological involvement presenting with chorea was diagnosed. She was given cyclophosphamide, methylprednisolone, acetylsalicylic acid (ASA) and valproate. Two years later she presented with acute right hemiparesis and aphasia. Cranial MRI showed acute infarction in left middle cerebral artery territory (Figure 1). Intravenous thrombolytic therapy was administered and her neurological symptoms improved. Extra and intracranial MR angiography was normal. Mitral valvular fibrotic thickening and patent foramen ovale with left-to- right shunt were detected on transoesaphageal echocardiography. She was on mycophenolate mofetil and methylprednisolone, warfarin was added.
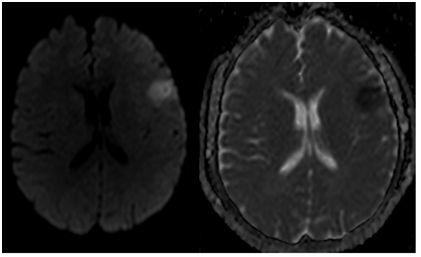
Figure 1 Left frontal acute ischemic lesion on diffusion weighted imaging (left) and hypointensity on the same area on apparent diffusion coefficient weighted imaging (right).
A year later she presented with acute abdominal pain, headache and transient dysarthria which lasted 1 hour. Her neurologic examination was normal. She had bicytopenia: Hemoglobin: 6.78 g/dl (11.7-15.5) and platelets: 120.000/mm3 (160.000-390.000). Coombs tests were negative, international normalized ratio (INR) was 2.74. Cranial computed tomography (CT) showed left frontotemporal sulcal hemorrhages,cranial MRI showed left dominant bilateral frontotemporal sulcal hemorrhages on fluid attenuated inversion recovery (FLAIR). Contrast enhancement on left dural convexity was suggestive of hypertrophic pachymeningitis (Figure 2). Cranial MR angiography and venography didn’t show any aneurysms and venous thrombosis. CSF findings were normal, oligoclonal bands were absent in serum and CSF. Acute phase reactants were elevated in serum and abdominal pain was considered as serositis and improved after 40mg/day intravenous methylprednisolone (IVMP) treatment for 5 days. SAH was considered to be a part of SLE disease activity facilitated with warfarin use and mild thrombocytopenia. Warfarin was stopped and she was discharged with ASA, mycophenolate mofetil and methylprednisolone.
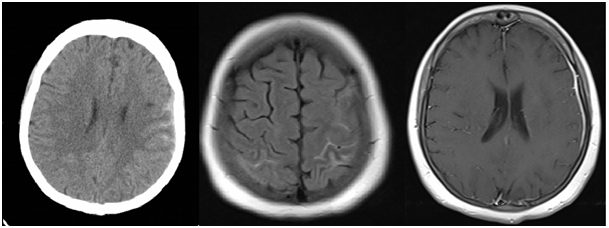
Figure 2 Hyperdense lesions on left frontotemporal sulci on cranial CT; convexal subarachnoid hemorrhage (cSAH) (left). Left dominant bilateral frontotemporal sulcal hyperintensities on FLAIR (middle). Contrast enhancement on left convexity dura on T1- weighted contrast image; hypertrophic pachymeningitis (right).
She was admitted with transient right hemiparesis 2years after the last episode andin the meantime warfarin had been restartedon the grounds of lupus anticoagulant (LA) positivity. Bicytopenia was evident (Hemoglobin: 8.4g/dl, platelets: 109.500/mm3), thrombotic thrombocytopenic purpura was excluded, iron and folate replacement were initiated. LA: 116 s (N: 30-38), anti-cardiolipin and anti-DNA antibodies were negative. INR was 3.22 and warfarin was stopped. Cranial MRI showed subdural hemorrhage in the interhemispheric fissure. Neighboring sulci were indistinct on T1 weighted imaging (WI) and hyperintense on FLAIR images. Falx cerebri was hypointense on Gradient Echo WI but neighboring sulci were not (Figure 3). 1000 mg/day IVMP was administrated for 3 days and continued with 40 mg/day. On the 8th day of clinical presentation she complained of headache, nausea and diplopia. Left 4th cranial nerve palsy findings were evident on neurological examination. Cranial MRI showed new hemorrhage on left cerebellar tentorium, contrast enhancement in the interhemispheric fissure and left cerebellar tentorium spreading through neighboring sulci (Figure 4), cranial angiography and CSF findings were normal dejecting a vasculitic process. Cerebral hemorrhages were considered to be a part of SLE disease activity facilitated with warfarin use and mild thrombocytopenia. Cyclophosphamide and rituximab were added. Her diplopia regressed over 6 months.
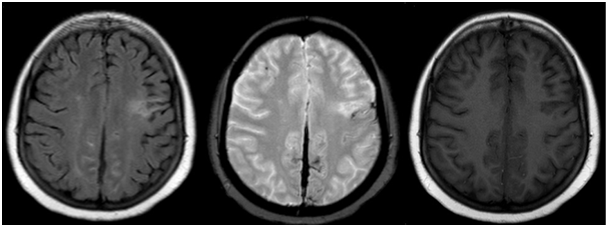
Figure 3 Subdural hemorrhage in the interhemispheric fissure. Neighboring sulci are hyperintense on FLAIR and previous ischemic stroke sequela is evident as left frontal hyperintense lesion (left). Falx cerebri is hypointense on Gradient Echo weighted image but neighboring sulci are not (middle). Neighboring sulci become indistinct on T1 weighted image (right).
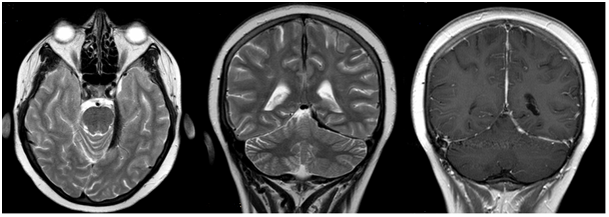
Figure 4 Subdural hemorrhage along left cerebellar tentorium on T2 weighted axial imaging (left). Hypointensities along interhemispheric fissure and left cerebellar tentorium on T2 weighted coronal imaging (middle). Contrast enhancement on interhemispheric fissure and left cerebellar tentorium spreading through neighboring sulci on T1-contrast coronal imaging (right).
3-20% of SLE patients may present with stroke. Stroke affects younger SLE patients and the risk of stroke increases to 57% with aging.1 Causes of stroke in SLE are cardiac embolism (especially patients with myocardial infarction, atrial fibrilation and non-bacterial endocarditis), arterial atherosclerosis, arteriolar disease (in patients with hypertension), thrombosis associated with antiphospholipid antibodies and rarely vasculitis.2
In 35-59% of SLE autopsy cases, cardiac valvular involvement was found and in most of these cases antiphospholipid antibodies were detected. In another autopsy study in SLE patients with neuropsychatric involvement, none of them had active intracranial vasculitis.3 Another study showed that non-inflammatory vasculopathy due to hyalinization of vessel walls and proliferation of endothelial cells and fibrin-thrombin clots are the most common causes of cerebrovascular diorders in SLE. Cause of the vasculopathy is not known well but endothelial damage induced by antineuronal antibodies or immune-complex deposits is suggested as a putative mechanism.4 There have been no prospective studies done on the treatment of stroke or protection from stroke in SLE.2 Using anticoagulants in SLE patients reduce the risk of stroke recurrence. It may be appropriate to use anticoagulants in SLE patients with cardiac valvular disease, transient ischemic attack or stroke. In retrospective studies ASA is found less effective in preventing stroke recurrence in SLE patients with lupus anticoagulants. Steroid is not recommended in SLE patients with stroke unless there are inflammatory lesions in cranial MRI.1 Our patient was admitted with ischemic stroke when she was 19. Mitral valvular involvement and patent foramen ovale were detected and warfarin treatment was initiated. But she presented with subarachnoid hemorrhage 2years later when she also had mild thrombocytopenia. Warfarin was stopped and ASA was initiated. In follow up, LA was positive and warfarin was reinitiated. Consequently, she presented with subdural hematoma when she had mild thrombocytopenia, again.
Ischemic stroke is more common in SLE but hemorrhagic stroke is also reported in cases of recurrent thrombocytopenia.1 Studies failed to show a relationship between subdural hematoma and disease activity of SLE and usually conditions facilitating hemorrhage were present. In a study, SLE patients with antiphospholipid antibodies who were on oral anticoagulants (OACs) had major bleedings including subdural hematoma; some of them were also using acetylsalicylic acid (ASA) and had hypertension.5 Another study showed that having LAs alone didn’t increase the risk of non-traumatic subdural hematoma and all patients with subdural hematoma also had risk factors which facilitated bleeding including thrombocytopenia, hypoprothrombinemia, OAC use, intracerebral venous hemorrhage, aging and head trauma.6 Interestingly it was shown that most of the patients didn’t use anticoagulants, most of them didn’t have antiphospholipid antibodies and presence of vascuilits in bleeding area was found in half of them with high disease activity of SLE.7 Another rare type of subdural hemorrhage is interhemispheric subdural hematoma and 91% of patients with this kind of hematoma were shown to have prior head trauma.8 Anticoagulant use9 and cardiac problems10 were also reported as rare causes of interhemispheric subdural hematoma but no patients with SLE were reported.
Our patient presented with transient right hemiparesis and cranial MRI showed interhemispheric subdural hematoma. During her hospital stay she developed diplopia, 4th cranial nerve palsy signs were evident and cranial MRI showed new hemorrhage on left cerebellar tentorium. There was contrast enhancement on the interhemispheric fissure and left cerebellar tentorium spreading through neighboring sulci. Cerebral hemorrhages were considered to be a part of SLE disease activity facilitated with OAC use and mild thrombocytopenia. To our knowledge, this is the first case with SLE and interhemispheric subdural hematoma reported in the literature.
Convexal subarachnoid hemorrhage (cSAH) makes up 6-7% of non-traumatic SAH patients.11˗12 The most common cause of cSAH in young patients is reversible cerebral vasoconstriction syndrome (RCVS) and cerebral amyloid angiopathy (CAA) in elderly patients.12 In a study on 30 patients with cSAH, over-anticoagulation was found to be the etiological factor in only 1 patient.13 In another study no etiological factors were identified in 20% of patients with cSAH and 66% of patients using antithrombotic drugs including ASA and OAC.11 In a study of 30 patients with cSAH thrombocytopenia and/or warfarin use were shown to be etiological factors in 3 patients, lupus vasculitis was shown in one patient.14
In a retrospective study on 258 patients with SLE, SAH was found in 10 patients and frequency of SAH in patients with SLE was shown to be 3,9%. In 5 patients SAH occured during SLE attack and frequency of SAH due to SLE activity was shown to be at least 1,9%. In autopsies of these patients who presented with SAH during SLE attack, no aneurysm, vasculitic process or atherosclerosis were shown in most of the patients and most of these patients were taking inappropriate treatment prior to SAH or they were not taking steroids or they had steroid resistant central nervous system (CNS) involvement of SLE. In most of the patients with inactive SLE and SAH, aneurysms were detected.15 In another study prevalence of SAH in SLE patients was shown to be 0,93% and only in 50% of the patients, aneurysms were detected.16 In these studies it was not mentioned whether SAH was cSAH or not. In patients without aneurysms; hemodynamic changes, coagulopathy, angiopathy caused by steroid induced atherosclerosis and anticoagulant use may account for SAH.16˗18
Our patient presented with sudden onset headache and transient dysartria; cranial MRI showed cSAH. Cerebral angiography didn’t show an aneurysm, vasculitic appearance or RCVS. Elevated acute phase reactants, abdominal serositis suggested that cSAH could be a part of SLE disease activity facilitated with OAC use and mild thrombocytopenia. It may be appropriate to use anticoagulants in SLE patients having cardiac valvular disease, transient ischemic attack or stroke. But hemorrhagic strokes can be facilitated with anticoagulant use and thrombocytopenia and may be a part of SLE disease activity.
We thank our patient RY for consenting to her case history to be published.
None.
None.

©2016 Atmaca, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.