Journal of
eISSN: 2373-6410


Case Report Volume 9 Issue 4
1Department of Neurosurgery, Mediclinic Al Noor Hospital, United Arab Emirates
2University of Western Sydney, Australia
3Medtronic MEA, India
Correspondence: Maher Mansour, Department of Neurosurgery, Mediclinic Al Noor Hospital, P.O.Box 46713, Abu Dhabi, United Arab Emirates, Tel 00971504456722
Received: July 20, 2019 | Published: July 30, 2019
Citation: Mansour M, Mansour YM, Mansour L, et al. Nucleus ventralis oralis anterior a potential target in deep brain stimulation for post anoxic dystonia? J Neurol Stroke. 2019;9(4):212-215. DOI: 10.15406/jnsk.2019.09.00378
Deep brain stimulation (DBS) effectiveness in secondary dystonia is still debated. Different targets have been utilized, thalamic (Vim), zona incerta, sub thalamic nucleus (STN), and globus pallidus internus (GPi) with generally disappointing clinical results. Structural lesions of the GPi, the target of choice in Dystonia, are found in some cases of secondary dystonia and prevent us from treating these patients with DBS implantation. We report the third published case of a patient with severe generalized post anoxic dystonia, associated with bilateral necrosis of the GPi, who showed significant improvement after DBS procedure in the thalamic Ventralis oralis anterior (Voa). The Ventral oralis anterior nucleus; pallidal receiving area in the thalamic nuclei could be an alternative target.
Keywords: post-anoxic dystonia, deep brain stimulation, thalamus, ventral oralis anterior, ventral lateral nucleus, secondary dystonia
DBS, deep brain stimulation; STN, sub thalamic nucleus; GPi, globus pallidus internus; Voa, ventrolateral thalamic nuclei; MRI, ventrolateral thalamic nuclei
Deep brain stimulation (DBS) of the thalamus (Vim), zona incerta, sub thalamic nucleus (STN), and globus pallidus internus (GPi) as well as pallidotomy (GPi) and thalamotomy (ventro-intermedio-medialis [Vim], ventro-oralis posterior [Vop]) have been reported to improve generalized or focal dystonia.1–15 Thalamic DBS for dystonia may increase the dystonic symptoms, especially when the Vim nucleus is the target of the stimulation.16 Targeting the thalamus was abandoned because several publications suggested that the Globus pallidum internus (GPi) is a better choice for stereotactic treatment in patients with dystonia.14,17–21 Structural lesions of the GPi might be an obstacle for the DBS electrode placement and stimulation; therefore, the Ventralis oralis anterior (Voa) nucleus (Ventral lateral anterior in the Anglo-American terminology), pallidal receiving area in the thalamic nuclei, could be an alternative target.22–25
There has been only two reports of the efficacy of DBS in post anoxic, dystonia with necrosis of the pallida, although this is one of the most frequent causes of generalized dystonia.23,24 We report the case of a patient with severe generalized post anoxic dystonia, and bilateral structural abnormalities of the GPi, who showed dramatic improvement 18months after thalamic Ventralis oralis anterior nucleus DBS.
Patient history
The patient was a 26year-old male, drug-abuser, and experienced a 3-day hypoxic coma, in unknown circumstances. During the following months, he gradually developed generalized dystonic movements with spastic paraparesis, which evolved during the next years to generalized dystonia, progressively worsening and confining at best the patient to the use of a wheelchair, but mostly bedridden. When he was evaluated at our institution, 13years following the accident; he had generalized dystonia in all four limbs mainly in extension, predominant torsional dystonia in neck with left laterocollis and retrocollis and opisthotonic position of the trunk. He had spasticity and dystonic upper limbs, bilateral wrist and fingers deformity (Figure 1).

Figure 1 Pre-operative condition showing opisthotonic position in the bed, with retro and laterocollis.
He was aphonic, communicating with his entourage with his right thumb and index finger only, but had apparent orientation to time, place and persons with coherent answers. Severe pain was present in the neck, left shoulder, wrists and sometimes in the ankles. He was unable to walk or stand for 10years, aphonic for 5years, fed through PEG tube since 3years and was totally dependent in all daily living activities.
Burke-Fahn-Marsden rating scale26 (BFMRS) score was: 104/120. A T2 weighted brain Magnetic Resonance imaging (MRI) scan showed hypersignal bilaterally involving both pallida, mostly affecting the most medial part (Figure 2). Treatment attempts with Trihexyphenidyl, Carbidopa-Levodopa, Carbamazepine, Clonazepam, Phenytoin and Baclofen were unsuccessful. Botulinum toxin injections provided some relief of the muscular contractions but rapidly became ineffective considering the importance of dystonic movements. The family gave informed consent for DBS surgery. As the main output of the pallidum is the ventrolateral thalamic nuclei (Voa) and the centromedian-parafascicular nucleus,30,34 we decided to target Voa for DBS and hoped it would act directly on the last distal part of the retroactive feedback to the motor cortex from GPi.
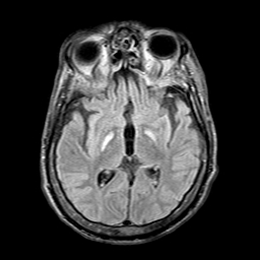
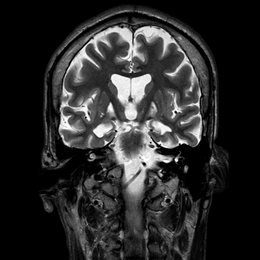
Figure 2 Pre-operative MRI showing selective necrosis of the Globus Pallidus interna, predominantly on the right side (above images are in radiological view).
Radiological exams
Stereotactic MRI was used to plan the target and trajectory (Siemens, 1.5 Tesla). All the sequences taken pre and post operatively confirmed to standard neuronavigation protocols- no gap, no interleaving, no angulations, square matrix and contiguous slices which were 1-2mm thick. 3D T1 sequences were acquired with an isotropic voxel (1mm) from the hard pallet to the vertex without contrast and with double dose contrast. 30 slices of 2mm thick T2 coronals were acquired; this sequence was centered at mid-commissural point (MCP). The length of AC-PC and width of third ventricle was measured at MCP. Height of the thalamus was measured on T2 coronals.
Surgical technique
Under general anesthesia, the patient underwent stereotactic implantation of quadripolar electrodes (lead model 3387, Medtronic, Minneapolis, MN) in the Voa bilaterally, using MRI-guided stereotactic surgery (Leksell-G frame, Elekta, Stockholm, Sweden) and macro electrode stimulation (using the Leksell Neuro-Generator, Elekta, Stockholm, Sweden) to rule out side effects caused by proximity to the posterior limb of the internal capsule under controlled general anesthesia.
Coordinates for Voa were, according to Guiot scheme,27 0.5mm posterior to the mid-commissure point (MCP) in order to reach the posterior part of Voa and anterior part of Vop, 10mm lateral and at the level of the anterior commissure-posterior commissure (AC-PC) plane (Figure 3). Only indirect visualization was possible as conventional MRI sequences cannot delineate different thalamic nuclei.
The planned electrode position was at the base of the Voa nucleus close to the Voa/Vop border (Figure 4). After carefully incising the dura at the marked point of entry a monopolar lesion making electrode (model 1017043 from Elekta, Stockholm, Sweden) was used to create a track for passage of the permanent lead. Impedance was monitored continuously to differentiate white or grey matter. Once the target was reached, macro stimulation was performed using the Leksell Neuro Generator up to supramaximal threshold of amplitude. Intraoperatively lateral fluoroscopy was used to verify the lead path and location. The actual position of the electrode was evaluated postoperatively by CT scan in stereotactic conditions and audited in the Leksell Surgiplan software (Version 10.1, Elekta, Stockholm, Sweden) (Figure 5). The difference between the planned and actual stereotactic target coordinates was up to a maximum of 0.5mm in lateral, anterior-posterior and vertical axis. The implantable neurostimulator (INS) Activa PC was implanted thereafter.
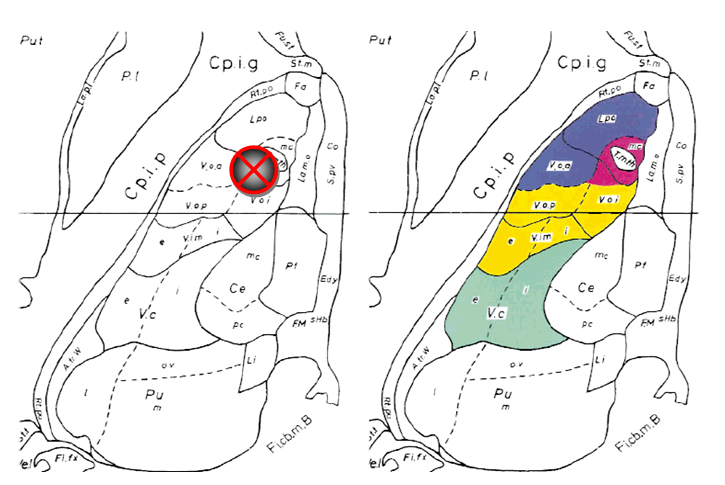
Figure 4 Left The horizontal plate from the atlas of Schaltenbrand & Wahren28 with Hassler’s29 nuclear outlines at 2mm above AC–PC plane, showing the position of the planned electrode.
Right: Same plate as at left with superimposed color-coded subcortical afferent territories extrapolated from comparable horizontal level of the monkey 24 thalamus from the atlas by Ilinsky et al.30 Somatosensory territory is shown in green, cerebellar territory in yellow, pallidal territory in navy blue, and nigral territory in burgundy. All abbreviations are as in the atlas by Schaltenbrand and Wahren.

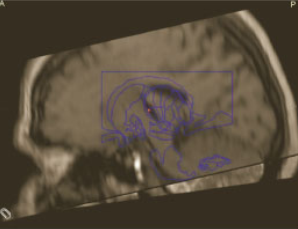

Figure 5 The correct position of the electrode was evaluated postoperatively by CT scan in stereotactic conditions and the Surgiplan software.
Initial programming was performed on the following day as no microlesion effect was observed. The initial postoperative stimulation parameters were 2.5volts, 185Hz, 250 microseconds, with monopolar stimulation on contacts 1 and 2 on the left Voa, 9 and 10 on the right side. Further adjustments of the stimulation parameters were performed during the follow-up period. Chronic stimulation was in the continuous mode. The amplitude was augmented bilaterally to 3.5volts.
Follow-up was performed at weekly intervals using the BFM rating scale. This scale is the sum of itemized scores obtained by multiplying the severity score by provoking factor by weight factor for each sub item for eyes, mouth, speech and swallowing, neck, arm and leg, and trunk, with a total maximal score of 120(26). Formal cognitive testing was not possible in this patient as he was unable to verbally communicate. During the first 2weeks, no significant changes were observed in his condition as compared with his pre-operative status.
After the third week, the patient was able to stand unaided and could walk with assistance, with only moderate persistent left proximal dystonia, retrocollis, laterocollis, and hesitation on changing directions (Figure 6). The BFM dystonia scale improved by 48% in total (104/120 pre-op, 54/120 post-op); disability scale improved from 13/30 to 6/30 and movement scale improved from 20/120 to 2/120. The functional status and the daily life activities were gradually improved after 18months postoperatively.
Deep brain stimulation is now worldwide approved for the treatment of refractory Parkinson’s disease, essential tremor and dystonia. It has been considered for new applications in a variety of other conditions including chronic pain, epilepsy and psychiatric disorders.1,2,9,11,17,32 The mechanism of action remains debated and the scope of clinical applications continue to fascinate the specialized physicians.
The globus pallidus internus was the first targeted structure for deep brain stimulation, to show significant improvement in patients with generalized dystonia without basal ganglia lesions.1,17,20,21,33 Usually the time delay, before onset of improvement, is about 3 months in observations with thalamotomy, Pallidotomy.4,5,10,12 and bilateral GPi-DBS.8,17
Structural brain lesions are common in secondary dystonia.31 It is possible that lesions of the GPi may result in loss of inhibitory pallidal projections to the thalamus and thus, provoke the hyperkinetic condition.23 In postanoxic dystonia, lesions in the GPi may impair the effect of GPi-DBS.
The main stimuli of the pallidum are through the Ventral oralis anterior nucleus (Ventral lateral anterior in the Anglo-American terminology) and the centromedian-parafascicular nucleus.23 Targeting the Voa for DBS could act directly on the last distal link in the retroactive feedback system to the motor cortex of the GPi.23,30,34 Only 3weeks after Voa-DBS, Our patient with generalized post anoxic dystonia had dramatically improved with important regression of dystonic movements, possible assisted gait, and partial autonomy in this patient who previously had used a wheelchair and was bedridden for more than 10years. The improvement has involved all areas of the body.
Side effects of the DBS have been difficult to estimate because of the difficulties to communicate with this patient.
To our knowledge, there are only 2 reported cases of post anoxic dystonia treated successfully with Voa DBS. The first case report was published in 2002 by Ghika et al.24 They have reported one patient with generalized post anoxic dystonia was first treated with DBS of GPi without significant improvement then with DBS of Voa. The patient showed a dramatic improvement after the second DBS procedure. The placement of the electrode in the Voa nucleus was confirmed by autopsy, as the patient committed suicide 4months after the procedure.
The second case, in 2009 published by Constantoyannis,23 the position of the electrodes in the Voa nucleus was confirmed by a postoperative MRI. The follow-up, over a period of 28months, revealed that the Voa nucleus offers, not only temporary, but satisfactory long-term results. Our report concurs with that of Ghika and Constatoyannis, that the Voa nucleus may well be an effective alternative when there is a damaged GPi. Our patient had presented with much worse preoperative condition, in comparison to those 2 reported cases, but had much earlier improvement, starting after 3weeks and was able to stand up alone at 3months and walked with assistance. The effects were stable throughout 18months.
Nevertheless, since these are only sporadic cases and reflect the experience on just three patients, further studies are deemed necessary to achieve a more informed perception as to who would benefit from Voa DBS. The interest to the Voa has raised several studies on primary35–38 and secondary dystonia,39 where, we know the Gpi DBS is not as effective as in primary Dystonia.
Our patient with generalized postanoxic dystonia dramatically improved after Voa-DBS. This suggests, in continuity with the 2 cases from the literature, that Ventral oralis anterior nucleus of the thalamus is a potential target in Deep brain stimulation for the dystonia treatment, together with GPi, especially when the basal ganglia are selectively damaged, such as in postanoxic dystonia.
None.

©2019 Mansour, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.