Journal of
eISSN: 2373-6410


Case Report Volume 9 Issue 4
Neurology Department, Texas Tech University Health Sciences Center, USA
Correspondence: Pranitha Reddy Arrabyru, Neurology Department, Texas Tech University Health Sciences Center, El Paso, Texas, USA
Received: June 20, 2019 | Published: July 9, 2019
Citation: Arrabyru PR, Khatri R, Maud A, et al. Intracranial stent placement for an acute ischemic stroke in a young patient with intracranial arterial dissection. J Neurol Stroke. 2019;9(4):185-187. DOI: 10.15406/jnsk.2019.09.00374
A 36-year-old Caucasian woman with a history of bipolar disorder was brought to the hospital after she was found down on floor. On examination, there was left hemiparesis, forced right gaze deviation and severe dysarthria. She was last seen normal six hours prior to the arrival and a computerized tomography (CT) of the head was unremarkable, except for a hyperdense right middle cerebral artery (MCA) sign. Subsequent magnetic resonance imaging (MRI) of the brain revealed early ischemic changes in the right hemisphere, less than one third of the right middle cerebral artery vascular territory. An MRI angiography showed a stump in the right M1 segment of the MCA. Vessel wall imaging MRI showed an intramural hematoma in the right M1 segment. The diagnosis of right M1 occlusion due to intracranial dissection was made and intracranial stent was planned. She had an excellent clinical and radiological outcome on follow up.
Keywords: middle cerebral artery, dissection, intracranial stenting, ischemic stroke
MRI, magnetic resonance imaging; MCA, middle cerebral artery; LVO, large vessel occlusion; ICD, intracranial arterial; CAD, cervical arterial dissection; DSA, digital subtraction angiography; NIHSS, national institutes of health stroke scale
Given the results of the recent acute ischemic stroke trials, mechanical thrombectomy is considered the standard of care for acute ischemic stroke related to a large vessel occlusion (LVO) within 6hours in the anterior circulation.1 Treatment can also be performed between 6 and 24hours when there is evidence of salvageable tissue.1,2 A meta-analysis of the five successful randomized controlled trials, revealed that mechanical thrombectomy using a stentriever fails recanalization in approximately 30% of all cases.3 Anatomical challenges (as in severe arterial tortuosity), tandem occlusions, large thrombi in the internal carotid artery, physical properties of the thrombi (soft/hard/organized), and underlying intracranial arterial disease like in atherosclerosis or dissection are possible aetiologies for the refractoriness.4
Arterial dissection is the most common cause of stroke in the young.5–7 Intracranial arterial (ICD) dissection particularly affecting the middle cerebral artery is a rare cause of ischemic stroke,8 compared to the cervical arterial dissection (CAD). Although digital subtraction angiography (DSA) is considered the gold standard diagnostic imaging, the typical findings of double lumen or intimal flap are seen in about 10% of the cases.9 The vessel wall imaging MRI (VW MRI) is emerging as a diagnostic tool to diagnose dissection, although at times it is difficult to differentiate between an intramural hematoma and an intraplaque hemorrhage.10 We present a case of an acute intracranial dissection that was successfully treated with primary stent placement.
A 36-year-old Caucasian woman presented to the Emergency Department after she was found down on floor, at her home. She had a history of bipolar disorder, no head trauma or vascular risk factors. She was last seen normal about six hours prior to her arrival to the hospital. Upon neurological examination, she was noted to have dense left hemiparesis, forced right gaze deviation and severe dysarthria, the National Institutes of Health Stroke Scale (NIHSS) was 10. A computerized tomography of the head demonstrated a hyperdense right middle cerebral artery (MCA) and no other obvious hyperdensities associated. An emergent magnetic resonance imaging (MRI) of the brain was performed (Figure 1). The MRI of the brain demonstrated early ischemic changes in the right hemisphere, less than one third of the right middle cerebral artery vascular territory, the MRI angiography showed an occlusion of the right M1 segment and an intramural hematoma was also noted in the right M1 segment. Knowing this information, the patient was emergently taken to the angio suite and the diagnosis was made of right M1 occlusion due to an intracranial dissection (Figures 2A–2C).
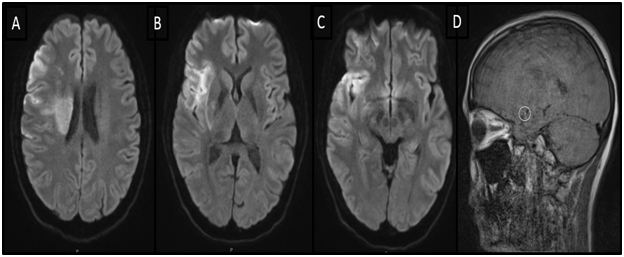
Figure 1 Magnetic resonance imaging (MRI). (A-C) Diffusion weighted imaging (DWI), axial view demonstrating early ischemic changes in the right hemisphere. (D) Vessel wall MRI, sagittal view showing an intramural hematoma in M1 segment of the right middle cerebral artery (MCA).
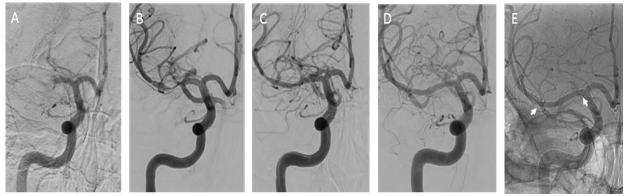
Figure 2 Digital subtraction imaging. (A) Anterior posterior view contrast angiogram demonstrating occlusion of the right M1 segment. (B) Double angiogram demonstrating the disrupted vessel. (C) Angiogram showing stent placement. (D) Follow up at 6 months. (E) Unsubtracted angiography demonstrating the stent at six months (arrows).
A 0.014-inch Synchro-2 standard microwire (Stryker Corporation, Kalamazoo, Michigan, USA) was used to reach (was advanced towards) the lesion and a Prowler Select Plus microcatheter (Codman, Raynham, Massachusetts, USA) was advanced over this guide wire. The wire was then removed and a double angiogram (via simultaneous microcatheter and guide catheter injection) demonstrated the area of stenosis/occlusion. An Enterprise 4.5x22mm (Codman, Raynham, MA) stent was then placed across the area of stenosis/occlusion. The patient was given an intravenous load of Integrilin (Eptifibatide) 135mcg/Kg and was loaded orally with aspirin 325mg and clopidogrel 300mg, to follow with daily aspirin 81mg and clopidogrel 75mg.
Clinically the patient improved on the table and was able to raise the left hemibody against gravity (MRC grading for muscle strength=4), we noticed complete resolution of the gaze deviation and improved dysarthria. A follow up CT head was performed on the day 1 (Figure 3). The patient was discharged home with outpatient rehabilitation and NIHSS=2.
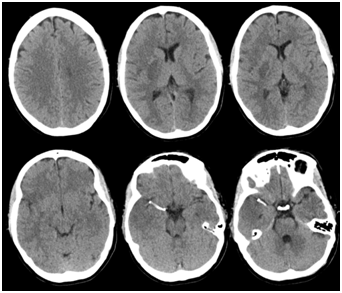
Figure 3 Computerized tomography (CT) head demonstrating intracranial stent in the right M1 segment of the middle cerebral artery, on day 1.
At three months, the patient had returned to her baseline with a modified Rankin score (mRS) of 0 and NIHSS of 0. Despite the lack of symptoms, the dual antiplatelet therapy was continued for about 6 months, time when she had a follow up angiogram (Figure 2D&2E).
We here present a successful case of an acute ischemic stroke due to an intracranial dissection treated with primary stent placement. Isolated middle cerebral arterial dissection (MCAD) is a poorly understood cause of stroke. The incidence is still not known.8 It should be considered when a young patient has a middle cerebral artery (MCA) territory infarct with stenosis/occlusion at the proximal segment of the MCA.11 The M1 segment of the MCA is most commonly involved in MCAD due to its proximity to the sphenoid wing.12,13 It is more commonly reported in Asians, and in the absence of obvious trauma, the etiology of most of the cases is spontaneous/idiopathic.7,12 Risk factors include history of trivial trauma, migraine, connective tissue disorders, and vascular risk factors like hypertension, hyperlipidemia, tobacco abuse, diabetes mellitus, and oral contraceptives.8,12
Isolated MCAD is more likely to result in ischemic stroke rather than hemorrhagic stroke syndromes.12 The proposed mechanism comprises the hemodynamic disturbance resulting from the stenosis or occlusion due to the arterial wall hematoma and/or the distal embolism of thrombi.6,8 The presenting symptoms of MCAD are variable, and the most common are headache, seizure, nausea, vomiting, tinnitus, and neurological deficits depending on the portion of middle cerebral artery involved.(12) Approximately 20% can present with no neurological deficits.8 Jisang Park et al. reported a proximal M1 portion of the right MCA dissection with active inflammation in a 33 year old Asian man who presented with recurrent left sided hemidystonia with smoking as the only stroke risk factor.14 The patient in this case was noted to have dense left hemiparesis, forced gaze deviation, and severe dysarthria due an occlusion of right M1 segment of MCA.
The diagnosis of MCAD depends on both invasive and noninvasive methods. The modalities commonly used include digital subtraction angiography (DSA), computed tomography/angiography (CT/CTA), magnetic resonance imaging/ angiography (MRI/ MRA), clinical and pathological evidence/ autopsy, transcranial Doppler (TCD).12 Every imaging technique has its own limitation. Although DSA is traditionally considered the gold standard for detecting dissections, there is no common agreement as to which diagnostic tool is most useful. The identification of the subintimal dissection is easier to diagnose with DSA, however the subadventitial dissection forming a pseudoaneurysm without narrowing the arterial lumen and flow impairment can be missed.10 Vessel wall magnetic resonance imaging (VW-MRI) appears to be superior. 10In addition in the presence of a complete vessel occlusion, the DSA has poor diagnostic value for dissection. In our case, the MRI of the brain demonstrated early ischemic changes in the right hemisphere. The VW-MRI showed an intramural hematoma in the right M1 segment of the middle cerebral artery, leading to the diagnosis.
The best therapeutic intervention for isolated MCAD is still unclear.8 The treatment strategies in the literature include the early use of antiplatelet agents, anticoagulation, conservative management, thrombolysis, surgery, and endovascular intervention.8,12,15 To date, no study has been performed to address the safety and efficacy of the different therapeutic alternatives. Some authors recommend against the use of anticoagulants as they consider those may promote progression of dissection and the intramural hematoma while others prefer anticoagulants as they slow down the progression of thrombosis.12 The safety of intravenous thrombolysis for intracranial artery dissection is unclear.12 Patients who are not responding to medical management, intracranial stent placement can be considered.6 Few cases are available in the literature about acute ischemic stroke in the setting of intracranial dissection. In Kim’s et al. study, in which a series of cases with intracranial dissection were studied, two patients presented with acute symptoms and had a self-expanding stent placed for acute symptomatic M1 dissection and internal carotid and M1 dissection, both experienced clinical improvement of the dissection-related stenosis, and mRS at 3months of 2 and 1, NIHSS of 7 and 0 at discharge.6
It is possible that intracranial dissection is the main cause of mechanical thrombectomy failure in young patients with stroke. As repeated passes of a stent retriever in the disrupted vessel may worsen the dissection and less likely to recanalize, early suspicion and recognition of intracranial dissection can lead the operator about a different approach, successfully as in our case.
The authors declare no conflicts of interests
Pranitha Reddy Arrabyru involved in manuscript writing; Rakesh Khatri, Alberto Maud involved in critical revision of the manuscript; Gustavo Jose Rodriguez involved in critical revision of the manuscript and also involved in patient care. All the authors have read and agreed to the final manuscript.

©2019 Arrabyru, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.