Journal of
eISSN: 2373-6410


Research Article Volume 3 Issue 4
1Department of Neurology, Al Ain Hospital, United Arab Emirates
2Department of Neurology, King Fahad Medical City, Kingdom of Saudi Arabia
3Division of Neurology, Mafraq hospital, United Arab Emirates
Correspondence: Khurram A Siddiqui, MBBS, FRCP, Designation: Consultant Neurologist & Epileptologist, Adjunct Associate Professor of Neurology, Department of Neurology, Medical Institute, Al Ain Hospital, PO Box: 16, Al Ain, United Arab Emirates, Tel 97137022578
Received: November 20, 2015 | Published: December 7, 2015
Citation: Siddiqui KA, Khalid E, Sinha S (2015) History versus. Epilepsy Monitoring Unit Evaluation for Diagnosing Seizures: A Concordance Study. J Neurol Stroke 3(4): 00100. DOI: 10.15406/jnsk.2015.03.00100
History is of paramount importance in the diagnosis of epilepsy. Accurate history can help select patients who require a comprehensive Epilepsy Monitoring Unit (EMU) evaluations, i.e. long term EEG monitoring, dedicated structural/functional neuroimaging and neuropsychological evaluation. A detailed history could help delineate seizure subtype and may obviate the need for a comprehensive evaluation, and be cost effective. We compared epilepsy diagnosis made in outpatients using historical information to comprehensive EMU evaluation. We reviewed seizure/spell diagnosis on consecutive patients made on the basis of history and compared to the final diagnosis following comprehensive EMU evaluation. This study was conducted in King Fahad Medical City, Riyadh, Saudi Arabia. Ninety Six patients (Male-51, Female-45) with mean (±SD) age 24(±10.1) years and duration of epilepsy 11(±9.7) years were recruited following outpatient evaluation probable diagnosis of focal seizure was made in 37, generalised in 29, Paroxysmal Non Epileptiform Seizures (PNES) in 6, and evaluation was inconclusive for seizure type in 24 patients. Following EMU evaluation diagnosis of focal epilepsy was made in 61, generalized epilepsy in 26, and PNES in 10 patients. Outpatient diagnosis was concordant to comprehensive EMU evaluation in 45% (43/96), which was 44% (27/61) for focal seizure, 42% (11/26) for generalised and 50% (5/10) for PNES. Concordance was seen in less than half of the patients recruited and was similar for all seizure subtypes. This study highlights the deficiency of accurate history probably related to the language barrier and or lack of health and educational literacy and signifies the importance of EMU evaluation in our population.
Keywords:Ictal event, Epilepsy, Epilepsy Monitoring Unit (EMU), Long term EEG monitoring, Neuroimaging, Neuropsychological evaluation, Seizure
Long term video electroencephalographic (VEEG) monitoring has been widely used for diagnosis, classification and management of seizures. It also prognosticates the patients presenting with recurrent seizures and monitor response to therapy.1,2 For most patients with epilepsy, “routine” EEG, bedside history and examination is sufficient to classify seizure subtype and initiate medical therapy. Also adding a brief video to routine EEG can increase diagnostic yield specially when there are frequent paroxysmal events. However, routine EEG has substantial limitations, as approximately 20% of patients,3 referred to a comprehensive epilepsy program because of medically refractory “seizures” don’t have epilepsy. These patients have either physiological or psychological disorders that may cause diagnostic confusion with epilepsy and result in unnecessary treatment with antiepileptic drugs. Moreover, initial EEG may not show epileptiform activity in more than 40% of patients to confirm the diagnosis of epilepsy.4 Paroxysmal events other than epilepsy cause problems in the diagnosis and management. Predominantly Psychogenic Non Epileptiform Seizures (PNES), which look like seizures, make it difficult for non-neurologists to differentiate what is real? Video EEG evaluation during the EMU admission has proven to be gold standard in identifying seizure as well as PNES. Previously published series,5-7 reported ≤55% diagnosis was psychogenic, 37% had epileptic events and 6% had both with VEEG monitoring. For PNES average delay in diagnosis is approximately seven years. The yield of routine EEG is 2.5-7% as compared to long term monitoring in EMU which is 50-70% for detecting seizures.7 With VEEG approximately 85% of patients can be given clear diagnostic conclusion which result in better management.8 It is observed that 37% of patients with the diagnosis of PNES had an epileptiform baseline EEG.9 VEEG monitoring i.e. ictal and inter ictal monitoring, performed in an epilepsy monitoring unit can help physicians identify ictal EEG patterns that may be necessary for classifying seizure types and determining surgical localization. VEEG monitoring may prove to be an essential procedure for helping physicians confirming diagnoses of seizure disorders, classify seizure types, and select surgical candidates who have intractable epilepsy.10 The main purpose of this study is to observe the significance of EMU in terms of reaching a correct diagnosis and localization of seizure onset by comparing it with history based diagnosis and localization. We set out to determine the frequency of agreement regarding epilepsy between history and EMU evaluation for diagnosis and localization.
We conducted a cross-sectional study at Epilepsy Monitoring Unit, Division of Epilepsy, Department of Neurology, King Fahad Medical City, Saudi Arabia. We did a consecutive sampling of adult patient admitted in EMU for spell evaluation. The duration of study was 6 months. We included patients with history of recurrent seizures, age from 12-80 years and we excluded: patients unable to give proper history and where collateral history was not available. For the sample size to be statistically significant in consensus with the hospital clinician we considered that at least 50% of the patients admitted to EMU will be correctly diagnosed by EEG. A sample size of at least 96 was required to have 95% confident interval, that the above stated true proportions of correctly diagnosed patient population is within the error margins of 10% (i.e. 40%-60%), using Z-statistics based confidence interval evaluations. Null Hypothesis: there was no difference between the diagnoses made on history as compared to the EMU evaluation with respect to epilepsy patients.
Appropriate approvals from ethics committee and the Institutional Review Board of King Fahad Medical City were obtained. The data collection tool was on a standard Performa. All patients prior to admission to EMU were reviewed for their initial diagnosis based on history. During EMU provocation was done in all patients, included photic stimulation, hyperventilation and sleep deprivation to provoke seizures/spells; both Video as well as EEG recording were synchronized for seizure/spell semiology. The changes on EEG and VEEG were recognised and documented on the Performa and final diagnoses was made based on these findings. The demographic variables of the patients along with any risk factors mentioned in history, were documented. EEG: done as outpatient along with the radiological findings on MRI brain done with epilepsy protocol were documented.
The variables (findings of diagnosis at pre and post evaluation) were arranged in a square table (array). The rows of the table correspond to the categories of diagnosis at pre-evaluation and the columns to the findings at post evaluation. Accordingly, the frequencies and proportions of the cross classification of the row and column variables were calculated. The main diagonal in this array reflects the agreement between pre and post diagnosis and the off -diagonal indicates the shift of diagnosis between pre and post evaluation. Test statistic for agreement used Kappa statistic (Cohen, 1960) which corrects for chance achieved agreement. An overall significance level of 0.05 was used for judging the statistical significance of the kappa statistic. Data entry, processing and statistical analyses was performed using SAS. Data was cleaned and descriptive statistics were carried out. Mean and standard deviation were completed for age.
In this study, we included 96 patients out of them 53% patients (n=51) were males and 47% (n=45) were females and their diagnosis were compared based on evaluation done in an outpatient setting and in EMU. All these patients were referred EMU for spell evaluation and characterization of seizures. The mean age of patients was ranging from 12 to 60 years with Age±SD (24±10.1) based on the inclusion criteria as shown in Graph 1. We also determined the duration of epilepsy of these patients, which showed us that average duration of epilepsy±SD (11±9.7) years. The patients were divided into 6 groups based on type of seizures and combination of seizures. We found that 46 patients out of 96 patients have no change in diagnosis with concordance in both evaluations of around 48%. So the diagnosis was altered based on EMU evaluation in 50 patients as presented in Table 1 & 2. The focal seizure group included a total of 37 patients in pre-admission evaluation and diagnosis was changed in 7 patients (generalized seizures 5, focal seizures and PNES 2) with concordance of 81% (as shown in Graph 2 & Table 3). So the diagnosis was changed in 18% of focal epilepsy group. The generalized seizure group included 29 patients in total and diagnosis was changed in 18 patients (focal seizures 15, PNES 2 and generalized seizures with focal seizures 1) with concordance of 38%. So change of diagnosis was maximally seen in generalized epilepsy patients (approximately 62%) after inconclusive group of patients. The PNES group included 6 patients in total and diagnosis was changed in 1 patient to generalized seizures with concordance of 83%. So the diagnosis was changed in 16.7% patients. The inconclusive group on pre-admission evaluation included 24 patients in total with change of diagnosis in all the patients to the categories already mentioned. The null hypothesis for this study was proved wrong as there was a significant change in the diagnoses of patients based on EMU evaluation when compared to history. The calculated p value is 0.0058 which is significant. The measured kappa value for the study is 0.2363 with 95% confidence interval limit of 0.1112 to 0.3614 which shows the concordance for two evaluations. All the patients were sorted out by the end of their evaluation in EMU so there were no more patients in the category of inconclusive seizures at the end of the stay in EMU.
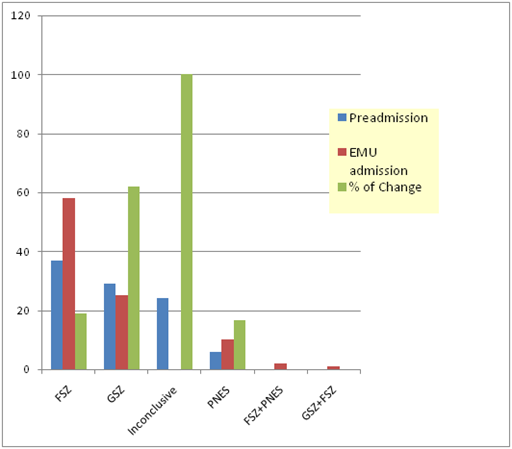
Graph 2 Change in diagnosis; FSZ: focal seizures, GSZ: generalized seizures and PNES: psychogenic non-epileptiform seizures.
Type of Seizures |
No. of Patients |
Focal Seizures (FSZ) |
37 |
Generalized Seizures (GSZ) |
29 |
Inconclusive |
24 |
PNES |
6 |
Total |
96 |
Table 1 History based diagnosis
Type of Seizures |
No. of Patients |
Focal Seizures |
58 |
Generalized Seizures |
25 |
Inconclusive |
0 |
PNES |
10 |
Focal Seizures & PNES |
2 |
Generalized Seizures & Focal Seizures |
1 |
Total |
96 |
Table 2 EMU Based Diagnosis
Seizures type |
Preadmission |
EMU diagnosis |
% o Change |
FSZ |
37 |
58 |
18.91 |
GSZ |
29 |
25 |
62.06 |
Inconclusive |
24 |
0 |
100 |
PNES |
6 |
10 |
16.66 |
FSZ+PNES |
0 |
2 |
0 |
GSZ+FSZ |
0 |
1 |
0 |
Table 3 Change in diagnosis; FSZ: focal seizures, GSZ: generalized seizures and PNES: psychogenic non-epileptiform seizures
Over the time there has been great advancement in the field of epilepsy in terms of diagnosis and management of patients with epilepsy. But overall there is not much change in the classification of epilepsy and epileptic syndromes. The role of genetics is also increasing for definitive diagnosis of epilepsy, especially genetic epilepsy syndromes. Imaging has also improved immensely to delineate the lesion in a better way so that more and more patients can be operated to resect the seizure focus for control of epilepsy. Newly selective surgical techniques are being developed with minimal possible damage to the anatomy of the brain. Now epilepsy neurosurgeons care more to prevent postoperative gliosis which can later develop into seizure focus. The role of EMU is expanding for definitive diagnosis and management of epileptic patients. It has become an inevitable diagnostic tool for epileptic patients referred to a tertiary care centre for diagnosis and control of seizures and other paroxysmal events.2,6,11,12 Selected patients are mainly referred for diagnosis, seizure classification and presurgical evaluations.8,10 It involves video EEG monitoring of patients to look for seizure semiology on video and to correlate it with EEG changes as outpatient EEG does not have high yield to localize the seizure focus which is possible with VEEG, it also provides help in planning surgery for selected patients. Ictal EEG is important for diagnostic classification of the paroxysmal disorders and selection of appropriate drugs for treatment. As a result, we can control seizures in a better and improve quality of life in epilepsy patients. The reported usefulness of EMU varies considerably from 19 to 75% due to different criteria in studies.4,10,13-15 In most of the patient’s neurologic history and with special attention to risk factors for epilepsy and outpatient EEG is sufficient. But descriptions provided by witnesses of the events (including family members) can be misleading and lead to errors in diagnosis and subsequent treatment. As we know that ictal EEG is superior to a routine interictal EEG to define seizure onset, outpatient EEG is not a very reliable indicator for seizure classification. Routine EEG results are altered by medication and sampling effect as well. The ictal EEG has its own limitations which should be kept in mind before proceeding for VEEG evaluations; may not be able to pick extra-temporal epileptic foci; patient may not have an ictal event despite he/she is admitted and had all provocations. Even repeating standard EEG can increase the yield to a certain extent.16
A challenge for a neurologist is how to diagnose patients with convulsive spells without any supportive electroencephalographic data, history is not very supportive and suspicion for psychogenic events is high. In such cases, VEEG is gold standard to rule out any ictal changes on EEG, as approximately 20% patients referred to tertiary care centres for the management of epilepsy are having psychogenic events.17-19 Usually it takes a long time to diagnose a patient with psychogenic events so proper management and referral to Neuropsychiatry is delayed even for many years and the patient will remain on AEDs with potential side effects. Another challenge in such patients is that sometimes they may have co-existent seizure disorder which can complicate the picture in such cases.20 Moreover, provocative measures like photic stimulation, hyperventilation and sleep deprivation can make seizures more severe and increase the risk of injury for the patient while stay in EMU, so family as well as the patient should be counselled before undergoing evaluation.21-23 Prolonged EEG monitoring is much superior to routine EEG in detecting seizures (50-70% with long-term monitoring compared with 2.5-7% with routine EEG studies). The change of diagnosis is observed around 24% to 47.5% in some studies.4,10
In our study, we found that EMU evaluation was significant as the diagnosis was changed in more than 50% patients which affected the management of these patients. The biggest change was observed in inconclusive group and generalized epilepsy group with change of diagnosis in 100% and 62% respectively. Also the several types of seizures were diagnosed in EMU evaluation: focal seizures with PNES and focal with generalized seizures.
We conclude that EMU evaluation has changed the way of evaluating epilepsy patients in terms of proper diagnosis that leads to improved control of seizures. This study highlights deficiency of accurate history probably related to language barrier, health and educational literacy and stigma associated with the diagnosis of epilepsy and signifies the importance of EMU evaluation in our population.
None.
None.

©2015 Siddiqui, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.