Journal of
eISSN: 2373-6410


Research Article Volume 2 Issue 5
1Department of Neurosurgery, University of Mississippi Medical Center, USA
2Department of Neurosurgery, The Houston Methodist Hospital, USA
Correspondence: Eugene V Golanov, M.D., Ph.D., The Houston Methodist Hospital, 656 Fannin Str., Suite 944, USA, Tel 713-441-8692, Fax 713-790-3075
Received: July 05, 2015 | Published: August 28, 2015
Citation: Shiflett JM, Parent AD, Britz GW, et al. Forehead stimulation decreases volume of the infarction triggered by permanent occlusion of middle cerebral artery in Rats. J Neurol Stroke. 2015;2(5):110-116. DOI: 10.15406/jnsk.2015.02.00067
Background: Universally present in animals diving response triggered by stimulation of the trigeminal nerve is known as “oxygen conserving reflex”. This powerful integrative response includes apnea, bradycardia, and blood flow redistribution targeted to protect the brain against hypoxia. We hypothesized that diving response triggered by forehead stimulation can be neuroprotective in rat.
Methods: Male Sprague-Dawley rats were anesthetized, intubated, and artificially ventilated while blood gases and body temperature were maintained at physiologic levels. Arterial pressure, heart rate and temperature and in some cases, CBF were monitored. The forehead skin selectively was continuously selectively cooled or electrically stimulated for one hour. Middle cerebral artery was permanently occluded and infarction volume was determined 24 hours later.
Results: Forehead cooling to +11 °C decreased infarction volume by 31% compared to control (58±14 mm3 vs. 87±12 mm3, corrected for edema, p<0.05). Electrical stimulation of the forehead decreased infarction volume by 65% (30±7 mm3 vs. 81±10 mm3 corrected for edema, p<0.01) while temporal muscle temperature remained unchanged. Blood gases, body temperatures were comparable in all groups. Forehead cooling alone triggered changes in arterial pressure and cerebral blood flow comparable to changes observed during diving response in rats.
Conclusion: Stimulation, temperature or electrical, of the forehead in rats decreases volume of the infarction produced by permanent occlusion of middle cerebral artery. Diving response may have neuroprotective component, and its activation can be beneficial for brain protection.
Keywords:Ischemic stroke, Diving response, Therapeutics, Electric stimulation, Hypothermia, Neurogenic neuroprotection
In spite of numerous investigative efforts, finding efficient therapy for the ischemic stroke remains elusive.1,2 Naturally occurring well-established phenomena of “preconditioning”3 and “postconditioning”4 suggest the existence of internal neuroprotective mechanisms.5 Recruitment of internal neuroprotective mechanisms provides attractive alternative for the existing methods.5-7 Currently, most often internal neuroprotective mechanisms or endogenous neuroprotection are activated by exposure either to sublethal damaging factors, such as ischemia, or by stimulation of selective brain structures.8-10
Here we explored neuroprotective potential of naturally occurring defensive mechanism known as diving response. Practically all mammals, including humans, during lifetime face situations with decreased oxygen supply such as diving, and developed a stereotypical response geared toward the surviving of hypoxia.11-13 Excitation of trigeminal (V1) or glossopharyngeal nerves, which innervate eyes, nose, and face, triggers complex systemic reaction, which is known as diving reflex or diving response.13-18 Diving response consists of highly coordinated increase in AP, decrease in cutaneous blood flow, expiratory apnea, bradycardia and increase of cerebral blood flow (CBF).11,19 Diving response seems to be present not only in humans or diving mammals but in all vertebrates.13,20,21 As diving response allows animals to survive in anoxic conditions longer than expected, it was called by Wolf oxygen-conserving reflex.22
In humans, just forehead cooling is sufficient to trigger “diving reflex”,18 which is oxygen conserving23 and suggested to be protective24 promoting survival after near drowning.25
We hypothesize that activation of the facial areas innervated by the first branch of the trigeminal nerve may have neuroprotective effect. Here we demonstrate that stimulation of the forehead in rats, area innervated by the trigeminal nerve,26 effectively decreases volume of the brain infarction triggered by permanent middle cerebral artery occlusion (MCAO).
Experiments were performed on adult male (350-400g) Sprague-Dawley rats. All experiments were performed in accord with the “Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research, National Research Council. Washington, DC: National Academy Press, 1996) and approved by University of Mississippi Medical Center IACUC, protocol # 1009. All surgical procedures were performed aseptically. After initiation, animals were intubated and anesthesia was maintained with 1.5-1.8% isoflurane in gas mixture of 20% O2 and 80% N2 using small animal respirator (SAR 830/P, ITC Inc, CA, USA). The depth of anesthesia was monitored by observing AP and corneal reflex. Femoral arteries were catheterized for continuous recording of AP (AD Instruments, CO, USA) and arterial blood gases monitoring (blood-gas analyzer, ABL5, Radiometer, Copenhagen, Denmark). Body temperature was monitored and maintained at normal levels using thermo pad feedback (908100-OPT-HB, TSE Systems MI, USA).
Rats were mounted in a stereotaxic apparatus (Kopf, Tujunga, CA, USA). To monitor the cerebral blood flow (CBF) the calvarium was exposed, and laser-Doppler flowmeter (Periflux PF3, Perimed AB, Jarfalla, Sweden) probe (tip diameter 0.45 mm) was placed over the parietal cortex after thinning the bone with a cooled drill to create rectangular window (3x4 mm). Subdermal forehead and temporal muscle temperatures were monitored continuously using thermoprobes (thermocouple 24 Ga, Physitemp, NJ, USA) introduced subcutaneously through the needle and recorded by data logger (ADInstruments, CO, USA).
To induce ischemic infarction we employed widely used distal occlusion model27 routinely used in our previous experiments.28-31 In short, MCA was exposed through the temporal craniotomy. Small cut was made in dura along the MCA. Thin stainless steel wire (0.2mm diameter) hook was inserted under the artery at the point just proximal to the lenticulostriate branches. The artery was lifted by the hook. The wire was carefully heated by the cautery while care was taken to avoid thermal damage of the underlying brain tissue. The artery was thus cauterized and proximal and distal segments were separated to prevent recanalization. The wound was closed. At the end of the experiment, anesthesia was discontinued. The animal was returned to the home cage. Twenty-four hours later, rat was deeply anesthetized and euthanized. Brain was removed, frozen and serially sectioned coronally every 400µm at 20µm thickness in a cryostat and stained with thionine for Nissl substance. Staining with thionine of Nissl substance is widely used to delineate the core of the infarction as early as 2 hours after the infarction.32 Due to chromatolysis in damaged neurons the core of the infarction is clearly brightened and demarcated from the surviving cell. The cross-sectional area of the lesion (characteristic sharp delineation of loss of Nissl-staining by damaged cells) was digitized using MCID-Elite software (Amersham Biosciences Corp, Piscataway, NJ, USA). The lesion volume was estimated by investigator blinded to the specific experiment. Infarction volume subsequently was corrected for edema (infarction volume* (volume of contralateral hemisphere/volume of infarcted hemisphere)).33
Cooling of the forehead was achieved by using specially designed cooling device. Cooling device was made of coiled copper tubing (diameter 3mm) with overall diameter of the coil of 2 cm. Water circulated through the coil was chilled to 10 °C by temperature controller (CL-100, Warner Instruments, LLC, Hamden, CT, USA). The cooling device attached to the holder was placed on the pre-shaved forehead skin only to touch it without excessive pressure.
Electrical stimulation (rectangular cathodal pulses, 0.5ms, 60µA, 25Hz, stimulator model 2100, A-M Systems, Seattle WA, USA) was delivered through two stainless steel needles (24GA) placed subcutaneously bilaterally along the lines connecting ear and eye.
Cooling or electrical stimulation was committed just before craniotomy for MCA occlusion (MCAO), lasted during the occlusion (10-15 min) and for one hour afterwards. After that, wounds were closed and animal was returned to the home cage. In control animals, temperature of circulating water was maintained at 36 °C for the same period of time. In another control group, needles were inserted as described but no electrical stimulation was delivered.
Statistics: Data were expressed as mean±SEM. Comparisons were made using ANOVA with least significant difference post-hoc analysis or paired samples T-test, when appropriate. Differences were considered significant at p<0.05.
Effects of the forehead stimulation on the volume of the infarction triggered by MCAO
Infarctions produced by occlusion of MCA: Volumes and distribution of the ischemic infarctions in both control groups (temperature or electrical stimulation control groups) were comparable and pooled together for the subsequent analysis. In the first control group (temperature stimulation control group, n=4) temperature of circulating water was adjusted to maintain subcutaneous forehead temperature at 36 °C. In the second control group (electrical stimulation control group, n=4) instrumented for the electrical stimulation, forehead temperature remained stable at 35±1 °C. After initiation of water circulation or needle introduction MCAO was performed. After MCAO MAP gradually increased maximally by 8% at 45 min (p>0.05) and returned to the baseline at the end of the one-hour observation period (Figure 1A). HR remained unchanged (Figure 1B). Temporal muscle temperature in both control groups was stable at 35±1 °C.
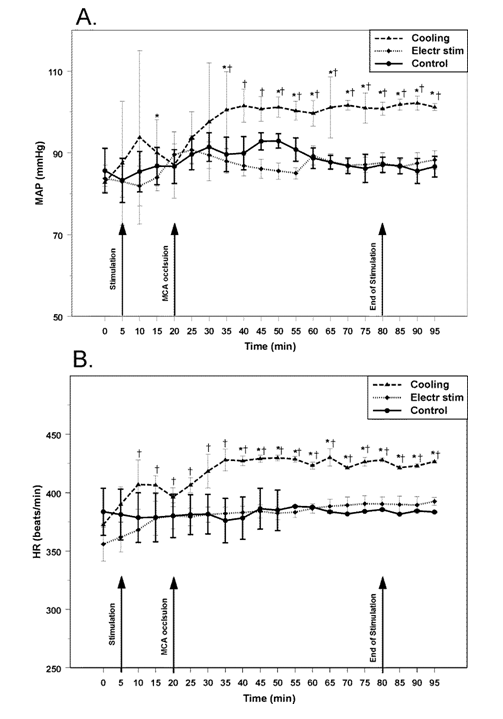
Figure 1 Changes in mean arterial pressure (MAP, upper panel) and heart rate (HR, lower panel) in rats in response to cold (Cooling, n=5); electrical (Electr stim, n=5); and sham (Control, n=8) stimulation of the forehead combined with middle cerebral artery occlusion. Arrows indicate beginning of stimulation, occlusion of middle cerebral artery and end of stimulation, respectively. * - p<0.05, compared to baseline; † - p<0.05, compared to other groups.
Volume of the infarction core produced by MCAO averaged at 85±11 mm3 (87±12 mm3 and 81±10 mm3, p>0.1, cooling and electrical stimulation control groups, respectively) as determined 24 hours after the occlusion (Figure 2). At the level of maximum damage, the lesion extended from primary motor cortical area to the piriform cortex, and to the outer sections of the caudate-putamen. In the rostrocaudal axis, the lesions involved large portions of the parietal, insular, temporal and occipital cortices (Figure 3,4).
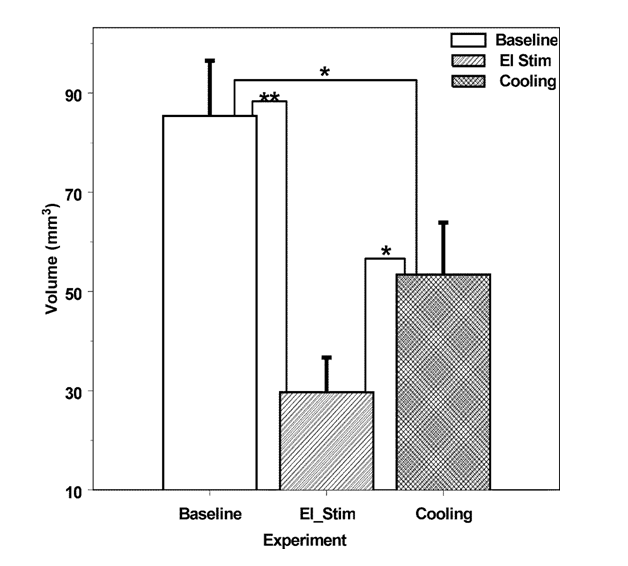
Figure 2 Comparative volume of the infarctions (corrected for edema) resulted 24 hours after permanent middle cerebral artery occlusion in control rats (Baseline, n=8), in rats in which forehead were stimulated with electric current (El Stim, n=5) and in rats, in which forehead was cooled to 11 °C (Cooling, n=5). * - p<0.05; ** - p<0.01.

Figure 3 Distribution of the infarction area at the crossections of the brain at different distance from the interaural line after middle cerebral artery occlusion in the rats which received sham (Control, n=8), temperature (Cooling, n=5) or electrical (El Stim, n=5) stimulation of the forehead as measured 24 hours after the occlusion. Distance indicates distance from the interaural line in mm. * - p<0.05, compared to sham stimulated group.
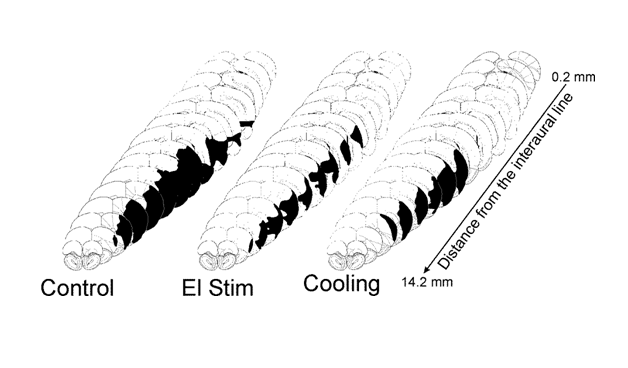
Figure 4 Representative distribution of the infraction (black areas) at different brain levels after permanent middle cerebral artery occlusion in rats received sham (Control), electrical (El Stim), or temperature (Cooling) stimulation of the forehead.
Effects of the forehead cooling on the focal ischemic lesions
Cooling of the forehead to subdermal forehead temperature of 11 °C significantly (p<0.05, n=5) reduced the volumes of focal ischemic infarctions by 31% to 58±14 mm3 (Figure 1). The salvage resulted from a decrease of the infarction volume prevalently in caudal regions of the ischemic lesion (Figure 3,4). During the cooling period rectal temperature remained at 36±1 °C, temporal muscle temperature was stable at 33±1 °C. MAP increased significantly by 8% (p<0.05) compared to the baseline after the initiation of cooling, increased further by 22% (p<0.05) in 20 min after MCAO and remained elevated during the period of observation (Figure 1A). HR increased after the initiation of cooling by 9% (p<0.05), increasing further to reach maximum of 15% (p<0.05) and remained elevated (Figure 1B) during the period of observation.
Effects of the subcutaneous electrical stimulation of the forehead on the focal ischemic lesions: Volume of the ischemic infarction core in rats (n=5), with electrical stimulation of the forehead averaged at 30±7 mm3 (corrected for edema): decrease of the infarction volume by 65% (p<0.01) (Figure 2) compared to matching control. The distribution of salvaged tissue in the forehead-stimulated rats was located at the periphery of the infarction, with most salvage located to the caudal areas of the infarction (Figure 3, 4). During the stimulation period rectal temperature remained at 36±0.5 °C and temporal muscle temperature was stable at 35±1 °C. MAP and HR changes were non-significant (Figure 1A,B).
Blood gases (PaCO2, PaO2, and pH) did not differ between groups: pH, 7.44±0.02; PaCO2, 38.6±2.4 mmHg; PaO2, 129.6±31.1 mmHg (mean±SEM).
Effects of forehead cooling on physiological variables
In the additional group of animals (n=4) we monitored the effects of selective forehead cooling on MAP, HR, and cortical CBF without MCAO. Following surgery and 10 minutes stabilization, cooling of the forehead was committed for 10 minutes; and rats were followed up for additional 10 minutes after the end of stimulation. Within 1 minute after the initiation of cooling, subcutaneous forehead temperature decreased to 11 °C. Forehead temperature returned to normal (35 °C) in 2 minutes termination of cooling. In parallel, MAP increased from 95±6 mmHg to a maximum of 101±6 (p<0.05) mmHg in 2 minutes. MAP remained elevated for the next 3 minutes and decreased slowly while remaining slightly above the baseline level for the rest of the experiment (Figure 5A). HR slightly increased by 2% (p<0.05) (from 385±6 to 389±5 beats/min), remained elevated for the next 3 minutes before returning to the baseline (Figure 5C). After cooling initiation, CBF rapidly increased to a maximum of 41±16% (p<0.05) in 5 minutes and started to decline returning to the baseline in 10 minutes (Figure 5B). In parallel with CBF increase, CVR (CBF/MAP ratio) dropped to a minimum of -23±7% (p<0.05) gradually returning to the baseline in 10 minute (Figure 5D). The period of cooling was limited to 10 minutes due to the spontaneous return of the CBF to the baseline. In control group of three similarly instrumented animals monitored for the same period of time (20 minutes), no changes in MAP, HR, CBF or CVR were observed.
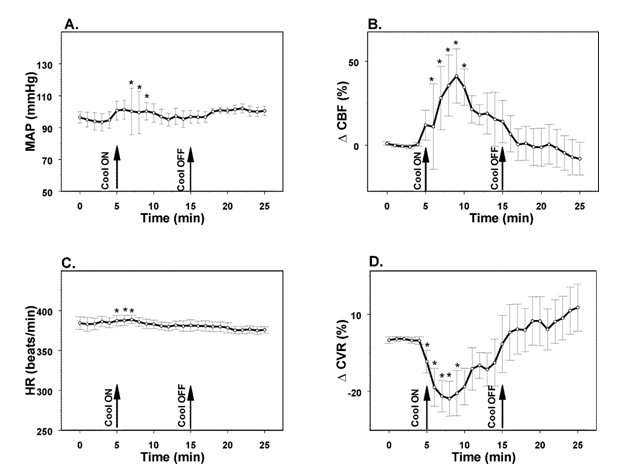
Figure 5 Changes in mean arterial pressure (MAP, A), heart rate (HR, C), parietal cerebral blood flow (CBF, B) and parietal cerebrovascular resistance (CVR, D) evoked by cooling of the forehead to 11 °C in rats. *- p<0.05, compared to the baseline, n=4. Arrows indicate onset (Cool ON) and end of cooling (Cool OFF) of the forehead.
Methodological considerations
The major goal of our experiments was to evaluate effects of trigeminal stimulation on the survivability of brain parenchyma under condition of permanent ischemia. It is suggested that the latter model may better reflect the therapeutic potential of treatment compared to ischemia/reperfusion model.34 Thus in our experiments we employed model of permanent MCA occlusion.
In our experiments, temperature or electrical stimulation of the forehead in rat significantly decreased, by 31% and 60%, respectively, the volume of the brain infarction triggered by permanent occlusion of MCA. The volume and distribution of focal ischemic infarctions in sham stimulated (control) rats were comparable to the infarctions described previously 31-36 indicating that our model is similar to generally used for evaluation of neuroprotective manipulations.
Decrease of the infarction volume core observed in animals in which forehead was stimulated was attributable to the preservation of brain tissue: it was still significant after correcting for edema, in agreement with previous observations33 and coincided with the periphery of the infarcted area.36 The possible protective effect of isoflurane could be also ruled out. Data on the protective effect of isoflurane are controversial; it can exert protective effect37,38 or aggravate neuronal damage.39 However, all our animals were under the same level of anesthesia for the comparable period of time and possible effects of isoflurane would be expected to affect results similarly. Nonetheless, we cannot exclude the possibility that stimulation-induced neural mechanisms of salvage could be affected by isoflurane differently.
The decrease in the infarction volume cannot be attributed to the differences in blood gases or body temperature, which were controlled and comparable in all groups of rats. Previously reported sympathetic activation accompanied by increase HR, MAP and blood catecholamines levels following MCAO due to the insular cortex ischemia was not observed in our experiments in control sham stimulated animals. The differences are probably due to the use of isoflurane in our experiments, which is known to suppress sympathetic activation and depress MAP.40-44
Comparison of electrical and cooling stimulation salvaging effects
The maximum salvaging effect in our experiments was afforded by the electrical stimulation of the forehead. Electrical stimulation employed in our experiments did not significantly affect MAP, HR, or forehead and temporal muscle temperatures, which remained comparable to such of control animals. These findings indicate that overall physiological state of the animals was not significantly affected by the local electrical stimulation of the forehead skin, and suggest that other than changes in MAP, HR or temporal muscle temperature factors were involved in neuroprotection.
Facial hairy skin in rats contains lanceolate, Ruffini and free nerve endings.26 Electrical stimulation at the parameters used excites multiple nerve endings, while cooling preferentially stimulates free nerve endings, which are thermosensors.45 In our experiments electrical stimulation, i.e. activation of nerve endings of different modalities, exerted most pronounce neuroprotective effect strongly suggesting that, first: hypothermia is not the main neuroprotective factor, and, second, excitation of other than cold sensitive nerve endings or combination of activation of nerve endings of multiple modalities provides maximum neuroprotection.
Cold stimulation of the forehead is accompanied by sympathetic activation, increase in MAP, and bradycardia.14,46 Observed increase in MAP and CBF in response to cold stimulation of the forehead is consistent with the diving-induced autonomic changes.11,18 Diving response is a powerful integrative response and includes apnea, bradycardia, and redistribution of blood flow,11,18,19 which is initiated by the excitation of the ophthalmic division of the trigeminal nerve. In humans dipping of the face into cold water is sufficient to initiate typical diving response.13,14 Our observations that temperature stimulation of the forehead in rats triggers autonomic changes resembling diving response and has neuroprotective effect suggest that diving response itself has neuroprotective component. In our experiments forehead cold stimulation elevated MAP and HR compared to other groups. While cold-induced sympathetic activation seems not to be affected by isoflurane,47 it’s vagolytic effect may explain the lack of bradycardia observed in our experiments.48,49 Another possibility is that bradycardia response was attenuated by artificial respiration, which is in line with observations that apnea potentiates diving-induced bradycardia.50
Importantly, electrical stimulation of the forehead produced even more pronounced neuroprotection without significant autonomic component. Lack of autonomic component in response to electrical stimulation at the parameters of the forehead used in our experiments can be a result of simultaneous excitation of medullary dorsal horn neurons of different modalities.51,52 It is conceivable that excitation of neurons other than those excited by nociceptive Aδ and C-fibers may counter balance autonomic changes triggered by the latter.51,53
It was suggested that diving reflex could be an important component promoting survival after near drowning.25 Our experiments strongly suggest that forehead stimulation, which is capable to induce physiological response resembling the diving response, is neuroprotective, and most probably has other than hypothermia mechanisms. First, cooling was highly localized to the forehead while temporal muscle temperature, which is used to estimate brain temperature,54,55 was not significantly affected. Second, electrical stimulation of the forehead, which did not affect temporal muscle temperature, afforded even more pronounced salvage than cooling alone. These findings allow concluding that excitation of the nerves of the ophthalmic division of the trigeminal nerve innervating forehead produces salvaging effect.
Possible mechanisms of forehead stimulation-induced neuroprotection
Mechanisms mediating forehead stimulation-induced salvage are presently unknown. Previously it was demonstrated that stimulation of the trigeminal nerve increases CBF probably through the CGRP-containing terminals innervating meningeal blood vessels.56-58 Selective cold stimulation of the forehead in our experiments produced significant elevation of CBF paralleled by decrease in CVR in accord with the previous findings.14,59,60 However, CBF returned to the baseline within 10 minutes after the initiation of cold stimulation suggesting that CBF increase is not the leading mechanism of neuroprotection as we suggested earlier.36
We suggest that other than CBF increase mechanisms, such as neurogenic neuroprotection,8,61 are involved in the diving response associated neuroprotection. Besides well-established neuroprotective effect of cerebellar fastigial nucleus stimulation which offers long lasting (up to three weeks)31 CBF- or cerebral glucose metabolism-independent neuroprotection62 the neurogenic neuroprotection can also be evoked by stimulation of subthalamic vasodilator area,63 and dorsal periaqueductal gray.28 Conceivably ventral thalamus64 and laterodorsal column of periaqueductal grey65 receiving projections from the medullary dorsal horn trigeminal inputs can activate innate neuroprotective mechanisms.
Endogenous neuroprotective mechanisms or neurogenic neuroprotection may include various factors such as opening of potassium channels, upregulation of uncoupling proteins and mitochondrial protein prohibitin, DNA repairing enzymes, and promote tissue repair mechanisms (recently reviewed (Wang et al., 2014).10
Experimental limitations
There are several limitations in our experiments. First, the infarction volume was determined only at one time point following the experiment (24 hours). In the future, it will be important to determine whether volume of the infarction remains smaller at longer times following the insult. Second, behavioral recovery evaluation needs to be performed to establish whether stimulation also promotes functional recovery early after the insult and at the later time. Third, optimal parameters for electrical stimulation need to be established. Fourth, it is imperative to establish time window following the MCAO during which forehead stimulation still is capable to decrease the infarction volume and improve recovery. These questions are currently being addressed in our investigations.
Here we demonstrate that cold or electrical stimulation of the forehead in the rat significantly decreases infarction volume following permanent MCA occlusion. Moreover, the electrical stimulation produced significantly more pronounced effect than cooling. We suggest that stimulation of the forehead activates endogenous neuroprotective mechanisms related to natural diving response universal to vertebrates. Activation of the endogenous protective mechanisms can offer significant neuroprotection in situations like stroke, brain trauma, and cardiac arrest and open the possibility to develop simple and useful methods for the treatment or prevention of brain damage. Just application of ice bag on the forehead in humans can simulate diving response and thus activate innate neuroprotective mechanism.66
The possible beneficial role of the diving response activation in humans is suggested by the reports of full recovery after people were submerged under water for prolonged periods of time (over one hour in some cases).67-70 Clinical observations suggest that survival after near drowning is not entirely attributable to the often-observed hypothermia: successful resuscitation was achieved at near normal body temperature67 and chances for resuscitation do not correlate with body temperature.71
Our observations may allow development of new simple approaches and methods to improve treatment of patients with brain damage.
The work was supported by NIH grant NS36154 and AHA grant 0250522N to E.G. and internal UMMC funds. Provide list of individuals who contributed in the work and grant details.
There are no conflicts of interests to disclose.

©2015 Shiflett, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.