Journal of
eISSN: 2373-6410


Research Article Volume 6 Issue 1
Faculty of Medicine, University of Belgrade, Serbia
Correspondence: Eleonora Dzoljic, MD, PhD, Specialist in neurology and clinical pharmacology Senior Research Fellow, Clinic of Neurology, Clinical Centre of Serbia. Faculty of Medicine University of Belgrade, Serbia, Tel (381) 11 3064222, Fax (381) 11 2684577
Received: November 17, 2016 | Published: February 13, 2017
Citation: Dzoljic E, Kostic V (2017) Fluoxetine does not Impair Motor Function in Patients with Parkinson’s Disease: Correlation Between Mood and Motor Functions with Plasma Concentrations of Fluoxetine/ Norfluoxetine. J Neurol Stroke 6(1): 00193. DOI: 10.15406/jnsk.2017.06.00193
Objective: Selective serotonin reuptake inhibitors are the most commonly chosen antidepressants in patients with Parkinson's disease (PD). The aim of our study was to assess the influence of fluoxetine (Flu) on motor functions in patients with PD.
Methods: In this prospective, controlled, open-label study, 18 patients with PD and mild depression (10 ≤ HDRS ≤ 23,) without dementia (25 ≤ MMSE) were treated with Flu. Both single and repeated dose effects of Flu were assessed on days 1-50. Plasma concentrations of Flu and norfluoxetine (NORFlu) were correlated with the results of selected motor function performance scores (UPDRS-motor score, FTT and PPT). Severity of PD, depression and dementia were evaluated using standard tests (HY, ADL, HDRS, MMSE).
Results: Steady-state for Flu/NORFlu was reached after 18 days of treatment. Such a plateau correlated with significant improvements in both scores of depression and Parkinson's disability (HDRS, UPDRS and ADL, respectively). In addition, FTT and PPT scores also increased until day 18, with further slight fluctuations around the plateau. Optimal motor performances correlated with Flu concentrations of approx. 60-110 microg/L.
Conclusion: Flu (20 mg/day) significantly reduced depression in PD patients while it did not impair their motor performances. Because substantial placebo effects may arise in studies of PD and depression, large, prospective, randomized, placebo-controlled clinical trials are warranted.
Keywords: parkinson’s disease, motor function tests, plasma concentrations of fluoxetine/ norfluoxetine
Depression is the most common and frequently disabling psychiatric condition in patients with Parkinson's disease (PD). Prevalence of depression in patients with PD varies from 7-76% depending on the assessment method.1 Such a depression is mostly persistent or recurrent. It may be accompanied with anxiety, cognitive impairment and may reduce effectiveness of antiparkinson’s therapy.2-5 The depression increases PD patients' disability and significantly reduces their quality of life. Consequently, approximately 50% of patients with PD receive antidepressant therapy.4˗7
The optimal treatment for depression in PD patients has not been established. Several antidepressants were tested in randomized clinical trials without sufficient statistical power (e.g. citalopram, sertraline, fluoxetine, amitriptyline and nortriptyline). Amitriptyline seems to be more effective than fluoxetine in PD patients with severe depression. However, it is not necessarily the first choice for treatment of depression in PD patients, according to the recommendations of the American Academy of Neurology.8 In addition, the adverse effects of amitriptyline such as orthostatic hypotension, sedation, cognitive and anticholinergic effects might preclude its use and increase the dropout rate in parkinsonians.1,9,20
On the other hand, selective serotonin reuptake inhibitors (SSRIs) are used as a first line treatment of depression 51% of the time.1,9,10 In postmortem studies of patients with PD depletion of 5-HT in the caudate as well as hypothalamus and frontal cortex was reported,11˗14 with preferential loss of 5-HT in the caudate compared with the putamen, but with relatively less loss of 5-HT (66%) than dopamine (98%).15 Imaging studies in vivo have also suggested depletion of 5-HT innervation to the striatum as measured via decreased 5-HT transporter binding.16˗18 The loss of striatal 5-HT in PD may be secondary to neurodegeneration within the raphe nuclei as Lewy bodies are seen in the raphe nuclei,19,20 associated with cell loss.21,22 Taucher et al.,23 were the first to demonstrate the pharmacodynamic action of the selective 5-HT transporter blocker fluoxetine in the human brain in vivo.23 Meyer et al.,24 showed that 80% 5-HT transporter occupancy was achievable with SSRI at therapeutic doses in a study in patients with mood and anxiety disorders.24 Apart from these drug-effects studies, it has been showed that recovery of central serotonergic system after SSRI therapy was associated with reduction of clinical symptoms in 18 depressive subjects using 123I-CIT and SPECT.25 All these findings of SSRIs-5-HT transporter occupancy in PET/SPECT studies clearly reflect the pharmacologically induced changes in serotonergic transmission.5,26
However, data on the efficacy and safety of SSRIs in PD are still lacking and sufficiently large scale randomised controlled trials are required. Although the introduction of SSRIs offers new opportunities for the treatment of depression in PD, these agents could produce extrapyramidal adverse reactions aggravating parkinsonism.1,10 While epidemiological studies have not suggested increased risk of worsening PD when SSRIs have been prescribed for depression,27 almost one hundred detailed reports on extrapyramidal adverse effects linked to SSRIs antidepressants have been published.28,29
The influence of Flu on motor performances in PD patients still remains to be clarified. Extrapiramidal side effects of Flu seem to be related to the exacerbation of Parkinson’s disability.30 However, it was also reported that Flu did not increase Parkinson’s disability either in retrospective31 or in prospective studies.32 Therefore, the authors argue for more systemic and controlled research examining the treatment of depression in patients with PD.1,33,34
The aimof this study was to investigate motor performances of PD patients treated with antidepressant Flu and to assess a possible correlation between mood and motor performance scores with plasma concentrations of Flu and its active metabolite, norfluoxetine (NORFlu).
Efficacy and tolerability of Flu was assessed in the prospective, 80 days, controlled, open-label clinical trial, with blind assessment. Flu was administered to 18 patients with nonfluctuating PD in the early Hoehn and Yahr stages (HY), I and II,35 accompanied with mild depression (Hamilton Rating Scale for Depression: 10 ≤ HDRS ≤ 23), without dementia (Mini Mental State Examination: MMSE ³ 25). These 18 patients were either a) de novo PD patients (PD0 group, N = 9), or b) PD patients who were on the stable antiparkinsonian treatment (PDt group, N = 9), without selegiline, rasagiline and/or dopamine agonists for at least two months prior to Flu.
Patients with secondary parkinsonism, those with the MMSE score < 25,36 history of stroke, neurological disorder other than PD, or any concomitant serious medical illness, and drug toxicity causing hallucinations, confusional episodes or delirium, were not included in the study. During the study, patients were not allowed to use neuroleptics, sedatives, hypnotics or other antidepressants, as well as drugs with potential extrapyramidal adverse effects. The study has been approved by the Ethical Committee of the Faculty of Medicine, University of Belgrade, Serbia. Before entering the study patients gave written informed consent.
All the patients were treated with two consecutive dosing regimens:
First, acute treatment with Flu: First day,patients received Flu, 20 mg per day, at 8 a.m. Evaluation of motor performances and blood sampling for Flu/NORFlu plasma concentration measurement were carried out immediately before the Flu treatment (day 1, 0 h), and 4 h, 6 h and 8 h after the administration of the drug. Flu was than withdrawn for three consecutive days. On the fifth day, patients received 40 mg of Flu at 8 a.m. and all the tests and blood sampling were repeated in the same order (day 5, 0-8 h after the administration of the drug). The pattern of blood sampling depends on Tmax for Flu, ranging from 4 to 8 hours after the single dose administration 37 (Figure 1, panel A).
Second, chronic treatment with Flu: In the same patients, regular Flu treatment was initiated (20 mg per day, at 8 a.m.) on day 6 after the beginning of such a therapy, and the motor performances were evaluated on days 11, 18, 50, and 80 (Steady state for Flu is reached after 18 days of Flu treatment) (Figure 1, panel B).
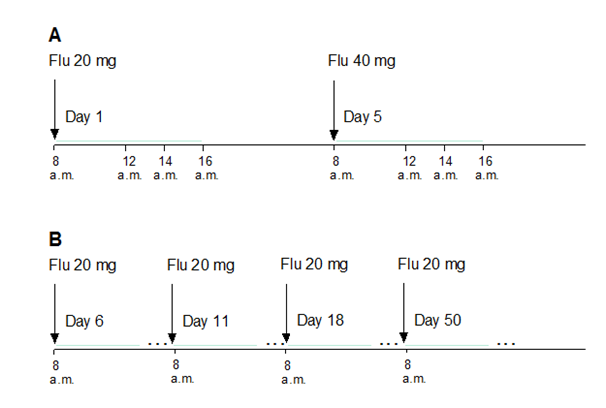
Figure 1 Design of the study: acute and chronic treatment of parkinsonian patients with Flu (panels A and B, respectively)
All the tests were performed in 18 out of 18 patients on days 11 and 18. Afterwards, 9 out of 18 patients were tested on day 50, and 8 out of 18 patients on day 80 (dropout rates of 50% and 44%, respectively). Two blinded refers evaluated severity of motor impairment using the Unified Parkinson's Disease Rating Scale (UPDRS-motor score,38), ADL (Schwab and England Activities of Daily Living Score) and computerized version of the quantitative motor test Finger Tapping Test (FTT,39) and the Purdue Pegboard Test (PPT,40). The current severity of depression was evaluated using the 17-item Hamilton Rating Scale for Depression (HDRS,41).
Bioanalytical method used for determination of plasma Flu and NORFlu concentrations was high performance liquid chromatography (HPLC) coupled with mass spectrometry (MS). The method used liquid chromatograph Therm Separation Products Spectra System (Autosampler AS3000, HPLC binary pump P2000, Degasser SCM1000), mass spectrometer with electro spray ionization source (Finnigan MAT SSQ7000 LC/MS - ESI System), Computer Digital UNIX Alpha Station 255. Recovery was very high, not less than 90.8 % for Flu and 80.2 % for NORFlu. Limit of quantification was 2.5 mg/L for Flu and 10 mg/L for NORFlu, and limit of detection was 1 mg/L for Flu and 5 mg/L for NORFlu. Correlation coefficient was 0.9993 (concentration range of 2.5-250 mg/L), and 0.9989 (concentration range of 10-250 mg/L), for Flu and NORFlu, respectively. Coefficient of variation, calculated for precision, was not higher than 8.33 % and 8.83 % for Flu and NORFlu, respectively.
Toxicity
EtCNeem was shown to be toxic to A. salina nauplii (LC50=288.46μg/mL). Through the extract saponification, it was observed that none of the obtained fractions caused the mortality of 50% of the A. salina nauplii (LC50 > 1.000μg/mL), in addition to not being toxic in adult zebrafih within 96 h of analysis (LD50> 5.0 mg/mL).
Antioxidant activity
F-EtOAc of EtCNeem showed higher antioxidant potential against DPPH (EC50 = 21.6 ± 0.07μg/mL). Pearson’s correlation coefficient (r) indicated that such antioxidant activity was correlated with the phenol (r = 0.4135) and flavonoid (r = 0.9924) contents in 41 % and 99 %, respectively. That is why this fraction was chosen for testing of anxiolytics.
Results are expressed as the mean ± standard error of the mean (S.E.M.) of N observations (descriptive statistics). Comparisons between groups were analyzed using Fisher's exact test, t-test, and one-way analysis of variance (ANOVA), when appropriate. In addition, correlation analysis, factor analysis, extraction method (principal component analysis), rotation method (Oblimin with Kaiser normalization) and trend analysis (fitting or least square method) were used.
All the patients were right-handed. Both groups, PD0 and PDt, had similar laterality of Parkinson’s symptoms (affected right side/affected left side = 6/3). Among 12/18 patients with affected right side, there was no significant difference between FTTr (FFT for right hand) and FTTl (FTT for left hand) scores, as well as between PPTr (PPT for right hand) and PPTl (PPT for left hand) scores (P = 0.66, and 0.89, respectively).
Among 6/18 patients with affected left side, FTTr was significantly better than FTTl (P = 0.03) and PPTr was significantly better than PPTl (P = 0.02). In addition, only PPTr score was significantly higher for left side-affected PD patients comparing to the right side-affected PD patients (P = 0.03). Age, gender and main clinical scores of PD0- and PDt-patients are shown in Table 1 & 2.
|
Group |
Age (years) |
Duration of PD (years) |
Previous Levodopa Therapy levodopa therapy levodopa therapy |
MMSE |
||
|
Duration (years) |
Dose (mg/day) |
|
||||
|
PD0 (N = 9) |
55.7 ± 3.0 |
2.7 ± 0.9 |
0 |
0 |
28.0 ± 0.6 |
|
|
PDt (N = 9) |
56.0 ± 2.7 |
3.6 ± 1.1 |
3.9 ± 0.9 |
458.3 ± 55.1 |
27.9 ± 0.9 |
|
Table 1 Baseline characteristics of patients with Parkinson’s disease: group of de novo patients without antiparkinson’s medication (PD0) and group with previous stable antiparkinson’s therapy (PDt) (mean ± S.E.M.)
PD - Parkinson’s disease; MMSE - Mini Mental State Examination.
Depressive symptoms were similarly reduced after 18 days of Flu treatment in both PD0 and PDt patients (Table 2, HDRS scores, P < 0.05). At the same time, Parkinson's disability was remarkably improved, especially in PDt patients (Table 2, UPDRS and ADL, P < 0.05, both).
|
Group |
HDRS |
UPDRS |
ADL |
|||
|
Day 1 |
Day 18 |
Day 1 |
Day 18 |
Day 1 |
Day 18 |
|
|
PD0 (N = 9) |
16.4 ± 2.1 |
10.4 ± 1.9¨ |
26.7 ± 2.9 |
23.6 ± 3.4 |
81.7 ± 3.8 |
85.0 ± 3.4 |
|
PDt (N = 9) |
13.6 ± 0.9 |
8.2 ± 1.1¨ |
29.0 ± 5.1 |
22.2 ± 4.6¨ |
82.2 ± 3.3 |
85.6 ± 3.4¨ |
Table 2 Staging of Parkinson’s disease: group of patients without antiparkinson’s medication (PD0) and group of patients with stable antiparkinson’s therapy (PDt), before (day 0) and on the 18th day of Flu medication (day 18, » steady state for Flu) (mean ± S.E.M.) PD: Parkinson’s Disease; HDRS: Hamilton Depression Motor Scale; UPDRS: Unified Parkinson's Disease Rating Scale; ADL: Schwab and England Activities of Daily Living Score.¨ - P < 0.05, day 0 vs. day 18 (Student's t-test for paired data)
Acute treatment with Flu: there were no remarkable changes in motor function scores (FTT, PPT) after the administration of 20 mg of Flu (day 1), or 40 mg of Flu (day 5) (Table 3). Groups PD0 and PDt differ only in FTTr scores at 0 h and 8 h after the administration of 40 mg of Flu (day 5).
|
Days of Flu treatment |
Group |
Day 1 |
Day 5 |
||||||
|
Parameter |
0 h |
4 h |
6 h |
8 h |
0 h |
4 h |
6 h |
8 h |
|
|
CFlu |
PD0 |
0 |
9.58 ± 1.51 |
11.44 ± 1.31 |
14.80 ± 0.80 |
3.24 ± 1.51 |
19.98 ± 3.30 |
23.19 ± 1.89 |
27.40 ± 2.06 |
|
(mg/l) |
PDt |
0 |
8.83 ± 1.02 |
14.76 ± 1.88 |
16.99 ± 2.28 |
5.87 ± 1.40 |
22.71 ± 3.39 |
25.60 ± 3.90 |
33.62 ± 2.87 |
|
CNORFlu |
PD0 |
0 |
0 |
0 |
0 |
0 |
3.57 ± 1.78 |
7.48 ± 1.78* |
10.48 ± 1.46 |
|
(mg/l) |
PDt |
0 |
0 |
0 |
0 |
2.57 ± 1.71 |
7.72 ± 2.02 |
11.95 ± 0.48 |
12.87 ± 0.49 |
|
FTTr |
PD0 |
5.11 ± 0.40 |
4.91 ± 0.45 |
5.20 ± 0.35 |
5.01 ± 0.43 |
5.44 ± 0.24* |
5.31 ± 0.28 |
5.19 ± 0.31 |
5.44 ± 0.30* |
|
PDt |
3.60 ± 0.53 |
3.55 ± 0.47 |
3.93 ± 0.43 |
4.14 ± 0.48 |
4.17 ± 0.44 |
4.16 ± 0.45 |
4.36 ± 0.43 |
4.16 ± 0.44 |
|
|
FTTl |
PD0 |
4.25 ± 0.41 |
4.34 ± 0.36 |
4.56 ± 0.36 |
4.57 ± 0.31 |
4.42 ± 0.24 |
4.46 ± 0.32 |
4.49 ± 0.32 |
4.83 ± 0.41 |
|
PDt |
4.00 ± 0.49 |
4.05 ± 0.40 |
4.12 ± 0.46 |
4.10 ± 0.46 |
4.21 ± 0.45 |
4.24 ± 0.42 |
4.15 ± 0.42 |
4.38 ± 0.40 |
|
|
PPTr |
PD0 |
10.33 ± 0.93 |
11.56 ± 0.96 |
11.56 ± 1.07 |
11.22 ± 0.85 |
11.33 ± 0.94 |
12.22 ± 0.81 |
11.78 ± 0.98 |
11.89 ± 0.92 |
|
PDt |
11.22 ± 1.10 |
11.56 ± 1.10 |
11.44 ± 1.09 |
11.78 ± 1.15 |
11.44 ± 1.08 |
11.67 ± 1.24 |
11.00 ± 1.27 |
10.89 ± 1.27 |
|
|
PPTl |
PD0 |
9.22 ± 0.66 |
9.89 ± 0.66 |
10.67 ± 0.67 |
10.44 ± 0.67 |
10.44 ± 0.75 |
10.67 ± 0.78 |
10.78 ± 0.88 |
10.33 ± 0.87 |
|
PDt |
11.22 ± 0.91 |
11.89 ± 1.32 |
10.78 ± 1.28 |
12.00 ± 1.18 |
12.00 ± 1.12 |
12.00 ± 1.30 |
11.78 ± 1.27 |
11.44 ± 1.16 |
|
Table 3 Changes in Flu and NORFlu concentrations, and motor function scores (FTT, PPT) during acute treatment with Flu (day 1: 20 mg; day 5: 40 mg) (mean ± S.E.M.) PD: Parkinson’s Disease; PD0 - de novo PD patients; PDt - PD patients with stable antiparkinson’s therapy; CFlu, CNORFlu - plasma concentrations of fluoxetine and norfluoxetine; FTTr, FTTl: Finger Tapping Test for Left and Right Hand; PPTr, PPTl - “Purdue Pegboard Test for Left and Right Hand; * - P < 0.05, PD0 vs. PDt
Chronic, treatment with Flu (20 mg/day, 80 days): plasma concentrations of Flu and NORFlu increased in a time-related manner (CFlu, and CNORFlu, respectively) (Figure 2).
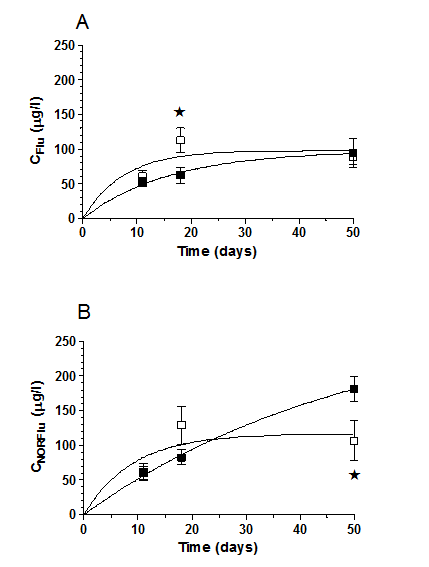
Figure 2 Changes of plasma concentrations of fluoxetine (CFlu) and its active metabolite norfluoxetine (CNORFlu) over time (panels A and B, respecively) in PD0 (o) and PDt patients (n), during chronic treatment with Flu (days 11-80, 20 mg/day). Each point represents the mean ± S.E.M. of plasma concentrations obtained from 9 separate PD0 or PDt patients. *P < 0.05, PD0 vs. PDt group.
Table 4 shows plasma concentrations of Flu and NORFlu as well as motor performance scores for the each group assessed during chronic treatment with Flu. Different patterns of changes were observed in PD0 and PDt patients. In the former case, a sustained increase in both CFlu, and CNORFlu was observed until day 18, i.e. the plateau was reached after 18 days of treatment. In the latter case, plasma concentrations continuously raised until the end of the observation period (day 50) (Table 4). CFlu were significantly higher in PD0 than in PDt group after 18 days of treatment (Figure 2, Table 4).
|
Days of Flu Parameter treatment |
Group |
Day 11 |
Day 18 |
Day 50 |
|
CFlu |
PD0 |
60.73 ± 7.31 |
112.21 ± 17.95* |
87.99 ± 9.88 |
|
(mg/l) |
PDt |
51.97 ± 6.52 |
62.34 ± 11.66 |
94.13 ± 20.54 |
|
CNorFlu (mg/l) |
PD0 |
62.17 ± 12.29 |
129.17 ± 27.43 |
106.51 ± 28.73* |
|
PDt |
60.80 ± 9.45 |
82.84 ± 11.22 |
181.74 ± 18.00 |
|
|
FTTr |
PD0 |
5.51 ± 0.26* |
5.56 ± 0.32 |
5.34 ± 0.45 |
|
PDt |
4.36 ± 0.42 |
4.47 ± 0.43 |
3.73 ± 0.80 |
|
|
FTTl |
PD0 |
4.62 ± 0.35 |
4.71 ± 0.32 |
4.78 ± 0.60 |
|
PDt |
4.27 ± 0.40 |
4.16 ± 0.43 |
4.11 ± 0.94 |
|
|
PPTr |
PD0 |
12.22 ± 1.06 |
12.89 ± 0.88 |
14.17 ± 0.53 |
|
PDt |
11.61± 0.97 |
11.83 ± 1.08 |
14.67 ± 1.76 |
|
|
PPTl |
PD0 |
10.89 ± 0.83 |
11.44 ± 0.90 |
11.81 ± 1.25 |
|
PDt |
12.06 ± 1.12 |
12.17 ± 1.19 |
14.50 ± 2.08 |
Table 4 Changes in Flu and NORFlu concentrations, and motor function scores (FTT, PPT) during chronic treatment with Flu (days 11-80: 20 mg/day) (mean ± S.E.M.) PD: Parkinson’s Disease; PD0 - de novo PD patients; PDt - PD patients with stable antiparkinson’s therapy; CFlu, CNORFlu - plasma concentrations of fluoxetine and norfluoxetine; FTTr, FTTl - Finger Tapping Test for Left and Right Hand; PPTr, PPTl - Purdue Pegboard Test for Left and Right Hand; * P < 0.05, PD0 vs. PDt
During chronic treatment with Flu, FTTr scores in PD0 group were continuously higher than in PDt group, reaching the significance on days 11 and 50 (P = 0.03 and 0.04, respectively) (Table 4). Such a difference was less pronounced regarding FTTl, PPTr and PPTl scores, never reaching statistical significance. Of note, the raise in CFlu between days 0 and 18 (the plateau) coincided with the increase in FTT and especially in PPT scores (Table 3 & 4).
Factor analysis reveals that influence of Flu/NORFlu concentrations increases over time (cummulative data from both PD0 and PDt patients; plasma samples were taken on days 0, 5, 11, and 18, six hours after Flu administration). The variance explained by the concentrations of Flu and NORFlu permanently increases from 13.9% (day 5) to 29.9% (day 11) and 37,6% (day 18) of cumulative variance (values of 89.4%, 84.9% and 91.8%, respectively). At the same time, influence of motor function scores decreases over time: variance explained by PPT and FTT scores of 75.5%, 55%, and 54.1% (days 5, 11, and 18, respectively).
PPT and FTT scores significantly correlated on day 11 (r = 0.62; P < 0.01). In addition, an inverse correlation was found between Flu/NORFlu concentrations and PPT-, but not with FTT scores, on day 18 (r = -0.70 and 0.48, respectively).
Gastrointestinal, cardiovascular side effects and/or insomnia, somnolence and excessive daytime sleepness as adverse reactions of Flu were not reported in the PD patients considered in the study.
Main results of our pilot study show that Flu treatment may alleviate depression in PD patients without deterioration of motor function scores. FTT, PPT and UPDRS-motor scores were even improved despite the parallel increase in plasma concentrations of Flu/NORFlu during first 18 days of the study.
Depression in PD must be properly diagnosed and treated.42 However, rare reports on the use of various antidepressants in PD patients offer controversial data on their safety regarding motor adverse reactions. Controlled clinical studies confirming the efficacy of Flu in PD patiens and assessing the risk-benefit ratio of such a therapy are still lacking.43
The broad therapeutic window for Flu is due to its highly variable pharmacokinetics.5,44˗46 Flu steady state is achieved approximately after 3 weeks (concentrations of approx. 110 mg/L). If plasma concentrations increase above 110 mg/L, the dosage should be adjusted accordingly. Factoranalyses indicates that mean Flu concentrations of approx. 60-110 mg/L have the most powerful effect on both PPT and FTT scores, which were significantly improved within that concentration range.
PPT and FTT are quantitative motor tests. While FTT more reflects motor speed, PPT is test for fine motor functions and coordination.40,47 Since all our patients were right-handed only among 6/18 patients with affected left side FTTr and PPT r were better than FTTl and PPTl, respectively, pointing to more efficient compensatory mechanisms in dominant hand.48,49
The pharmacological profile of fluoxetine is unique among the antidepressants used in PD patients. Fluoxetine is both SSRI agent and a 5HT2C antagonist.50 Recent investigation confirmed that 5HT1A agonists and 5HT2C antagonists could be important features in treatment of PD. In particular, 5HT2c receptors seem to tonically inhibit dopamine release from all three major dopaminergic pathways. Accordingly, 5HT2c antagonists could block such an inhibiton, especially in the terminal regions of the nigrostriatal and mesolimbic pathways.51
Additionally, 5-HT2c receptors are selectively located within the substantia nigra pars reticulata (SNr) and medial globus pallidus (GPm) and 5-HT via 5-HT2c receptors is excitatory in the SNr,52˗55 which may contribute to the increased activity of these regions in PD. Systemic administration of selective 5-HT2c antagonists to 6-hydroxydopamine-lesioned rodents potentates the antiparkinsonian action of dopamine D1 and D2 agonists,56,57 which is an action mediated via 5-HT2c receptors in the SNr.56 Thus, 5-HT2c receptor antagonists may improve parkinsonism and drugs with 5-HT2c receptor antagonist action, such as fluoxetine, are unlikely to worsen PD.57
The pathophysiological mechanisms involved in mood disturbances in PD remain complex. Serotonergic dysfunction has been postulated as such systems are involved in mood disorders in non-PD and the raphe nuclei, as well as hippocampus and prefrontal cortex, appear to be the primary sites affected.58,59 Moreover, transcranial ultrasound studies have suggested an association with reduced brainstem raphe echogenicity and nigral hyperechogenicity in patients with depression preceding PD onset compared with nondepressed patients with PD.60 As the PD disease progress, Lewy bodies occur with the rostral raphe, thalamus and limbic and cortical regions,15˗22,61 which may result in the mediating of mood disturbances in PD.23˗26
In depression associated with PD, PD-specific pathology, with multiple transmitter deficiencies in mesocortical monoaminergic systems, plays a major role. This includes the mesocorticolimbic dopaminergic projection as well as mesocortical noradrenergic and serotonergic projections. Corticolimbic noradrenergic denervation through cell loss in the locus coeruleus and serotonergic deneravtion via serotonergic cell loss in the raphe nucleus are also likely to be important.11˗15,22˗26, 62 Postmortem evidence has shown a lower density of neurons in the dorsal raphe nuclei in depressed versus nondepressed patients with PD22 and cerebro-spinal fluid measurement in vivo have shown reduced serotonin metabolite (5-HIAA) levels in depressed patients with PD.63 A 11C-DASB PET study in seven patients with PD with untreated depression showed elevated serotonin transporter binding in the prefrontal cortex compared with non-PD-matched controls.64 Recently, Politis et al.,65 has reported that the patients with PD with the highest scores for depressive symptoms showed significantly increased 11C-DSAB binding in the amigdala, hypothalamus, caudal raphe nuclei and posterior cingytlate cortex compared with those patients with low depression scores, though not compared with healthy controls. The11C-DSAB binding values in other regions, including the anterior cingulate cortex, caudate, insula, prefrontal cortex, putamen rostral raphe nuclei, thalamus and ventral striatum, were similarly decreased in patients with PD, irrespective of their depressive symptoms scores, compared with the healthy controls. This study demonstrates that depressive symptoms in antidepressant-naïve patients with PD are associated with relatively higher serotonin binding in raphe nuclei and limbic structures. The relative increase in serotonin transporter binding in these regions could reflect either lower extracellular serotonin levels or a disease-related loss of presinaptic serotonergic neurotransmission in contributing to the pathophysiology of PD depression.62,65
The phenomenology of depression in PD is also different from that in patints with non-PD with less anhedonia and feeling of guilt.66 While aetiology of depression in Parkinson's disease is unclear (biochemical changes, psychosocial factors and situational stressors have all been implicated), it has an adverse effect on the quality of patients' lives and doctors should ensure that it is diagnosed and properly treated.1,4,5,67
Therefore, along with improvement on parkinsonian quality of life due to antidepressant activity of SSRI, symptoms such as bradikinesia, hypomima, hypophonia that overlap between depression and parkinsonism could ameliorate because an improvement of mood symptms.1,9,10 Evenmore, Suzuku et al.,68 suggested that SSRIs such as fluoxetine potentially are therapeutic drugs for non-motor symptoms as well as motor symptoms in patients with PD, since fluoxetine can reverse the downregulation of cell proliferation in the subgranular zone by the unilateral 6-hydroxydopamine lesion.
All these various mechanisms could explain why the improvement in Parkinson's disability scores in our patients coincided with an increase in plasma Flu and NORFlu concentrations during the first 18 days of antidepressive treatment.
Another question is to assess the possible difference between PD0 and PDt patients' response to Flu treatment. The beneficial effects of Flu on motor symptoms of PD patients seem to be more pronounced in PDt group (UPDRS and ADL scores, Table 2). In addition, PPT scores were mostly higher in PDt patients during chronic treatment with Flu increasing continuously by the end of the study (day 50). However, the antidepressive efficacy of Flu was similar in both PD groups (HDRS, Table 2). Also, the statistical significance was rarely observed between those groups regarding motor function scores; FTT values were even somewhat higher in PD0 patients on days 11 and 50 (Table 4).
According to group of Taylor,69 depressive symptoms precede those of motor dysfunction in 12-37% of patients with PD. On the other hand, algorithms for treating depression in PD suggest that optimal antiparkinsonian treatment should precede the administration of antidepressants 1, e.g..70 Our results support such an approach only partially: PD0 and PDt groups did not differ in their response to antidepressive therapy, while the influence of Flu on motor functions scores was not consistently related to the pretreatment with antiparkinsonian drugs. Nevertheless, successfull treatment of PD before the administration of antidepressants may diminish overlapping between depressive symptoms and core Parkinson's diasease symptoms.1
In the present study, we failed to observe any deterioration in motor performance scores of patients with PD that was related to the increase in plasma Flu and NORFlu concentrations. A slight improvement was even observed in all the scores (UPDRS, ADL, FTT and PPT). Similar results were obtained with citalopram, which improved mood but did not decrease motor performance scores in PD treated with levodopa; at the same time, citalopram improved the parkinsonian dysability, bradykinesia and finger taps after one and four months of treatment, both in patients with and without depression.71,72 Also, Weintraub et al.,43 reported that escitalopram was well tolerated, but produced only a partial response in the treatment of major depression in elderly PD patients (mean age of 72.1 years). Two open-label studies suggested that sertraline reduced depression in PD patients, with additional beneficial effect on anxiety, without influencing motor function.73,74 In another open-label study with paroxetine (20 mg/day) given to 33 nondemented depressed PD patients during 6 months, Ceravolo et al.,75 reported significant improvement of depression, as evaluated by HDRS, without influence on parkinsonian symptoms. In only one patient fully reversible worsening of tremor was observed. However, paroxetine frequently may induce tremor as an adverse effect, with a prevalence of 1% to 2%. Chung et al.,76 reported that short-term paroxetine treatment did not alter the motor response to levodopa in patients with PD.
On the other hand, in two retrospective studies worsening of motor symptoms was observed in only small number of PD patients treated with SSRIs.77,78 In a prospective study comprising 65 depressed PD out-patients treated with paroxetine (10-20 mg/day) for at least 3 months, two out of 52 patients who completed the study (3%) experienced worsening of parkinsonian symptoms.79 However, van de Vijver et al.,80 observed that the start of SSRI therapy in levodopa users was followed by a faster increase of antiparkinsonian drug treatment. Gony et al.,81 in recent study failed to find any significant difference in the occurrence of serious extrapyramidal symptoms between different classes of SSRI antidepressant drugs in patients with PD treated with dopaminergic antiparkinsonian drugs. According to the results of several studies e.g. ,82,83 including our results with Flu, it seems that the benefit of SSRIs outweigh the potential problems due to adverse effects and that they may be considered to be the rational choice in the treatment of depression in PD.
This pilot study suggests that Flu in a dose of 20 mg is effective and well tolerated antidepressant in patients with Parkinson’s disease. In addition, Flu improved motor function scores in PD patients and such an improvement was observed in parallel with the increase in plasma Flu and NORFlu concentrations. Also, the effects of Flu were similar in de novo PD patients and in those already treated with antiparkinsonian medications.
There are several limitations of the study: it was an open-label study without randomization and recruited small number of patients. As with all nonradomized, open-label trials at tertiary research centres, many non-specific factors, such as relatively long duration of symptoms in de novo PD patients, may have influenced the results. However, the quantitative evaluations of motor functions using FTT and PPT significantly improved objectivity and validity of our findings. The observed dropout rates (50% and 44% on days 50 and 80, respectively ) are high but fit to the range observed in clinical trials in depression.84 Therefore, our results would allow an optimal design for further large, prospective, randomized, placebo-controlled clinical trials that are necessary to evaluate the efficacy and safety of SSRI antidepressants and allow the development of evidence-based guidelines.
This work was supported by the Ministry of Science, Republic of Serbia (Project No. 175090).
None.
None.

©2017 Dzoljic, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.