Journal of
eISSN: 2373-6410


Mini Review Volume 7 Issue 5
1Faculty of Biology of Lomonosov Moscow State University, Russia
2University diagnostic laboratory, Russia
3University Headache Clinic, Russia
4Department of Neuroscience, I.M.Sechenov First Moscow State Medical University, Russia
5Centre of Theoretical Problems of Physico-Chemical Pharmacology, Russia
6Department of neurology and neurosurgery, I.M.Sechenov First Moscow State Medical University, Russia
Correspondence: Eugene Klimov, Department of Genetics, Biological Faculty of Lomonosov Moscow State University, 119234, Moscow, Lenin Hills, 1-12, Russia
Received: August 21, 2017 | Published: September 27, 2017
Citation: Klimov E, Rudko O, Naumova E, Tretiakov A, Sobolev V et al. (2017) Familial Hemiplegic Migraine Type I: The Molecular Signaling Pathway. J Neurol Stroke 7(5): 00249. DOI: 10.15406/jnsk.2017.07.00249
Migraine is a multifactorial disease, manifested by intense bouts of recurrent headaches. Molecular mechanisms of migraine attack are not clear. In this study, we carried out the analysis of molecular processes in the pathogenesis of a rare hereditary form of migraine - familial hemiplegic migraine type I. Constructed hypothetical signaling pathways allowed us to understand the causes of a migraine attack and identify key molecules and signaling pathways for further experimental and clinical studies.
Keywords: familial hemiplegic migraine type I, fhm1, signaling pathways
Migraine is a multifactorial socially significant disease, manifested by intense bouts of recurrent pulsating headaches. There are three main theories describing migraine pathogenesis: vascular theory, neurogenic theory and trigeminovascular theory. Genetic predisposition to migraine is proved by epidemiological and genetic studies. Three forms of migraine with monogenic inheritance are well-known nowadays: familial hemiplegic migraine (FHM) of I, II and III types. But no genes or gene combinations associated with “common” migraine are known. Building a map of molecular signal pathways leading to a migraine attack may help to understand the causes of attacks and reveal key molecules and pathways. At the moment lack of information on genetic determinants of migraine and lack of animal models of “common” migraine makes it difficult to choose a starting point for a signal pathway. Because of this we have chosen FHM1 as a such point. FHM is a rare (0.003%) and severe form of monogenic migraine with aura which is characterized by the development of muscle weakness during aura. It is considered that the mutations in CACNA1A gene which encodes the main subunit of voltage-dependent calcium channels (VDCCs) (Cav2.1) cause the development of FHM1.
Main function of VDCCs is modulation of stimulating neurotransmitters release in neuromuscular synapse as well as in central synapses of cerebellum, brain trunk and brain cortex predominantly.1 More than 60 mutations which may lead to different phenotypical forms varying from pure FHM1 to FHM1 accompanied by cerebellar ataxia of varying severity or severe brain edema leading to fatal coma are known nowadays in this gene.2˗4 Mutations in CACNA1A gene may cause diseases not linked to FHM1 including episodic ataxia type 2,5 progressive ataxia,6 spinocerebellar ataxia type 67 and different forms of epilepsy.8 The development of FHM1 is mainly caused by missense mutations in CACNA1A gene (50-70% of families).9 In 40% of families with FHM1, a mutation leading to Thr666Met amino acid substitution was found (here and further the location of a substitution with a number between two amino acid names was marked).10 Arg192Gln mutation causes pure form of hemiplegic migraine, while Ser218Leu causes malignant form with the hemiplegic migraine attacks developing after any head injury and often accompanied by coma.11 Meanwhile Arg192Gln homozygotes may show normal phenotype, Ser218Leu heterozygotes are characterized by ataxia and high risk of sudden death due to severe epileptic seizures and cerebral edema.11,12 It was shown on the cell models that different types of mutations of CACNA1A gene in FHM1 cause different variants of channelopathies - a type of diseases characterized by ion channel function disturbance, changes in its structure and kinetics,13˗16 leading to increased Ca2+ ion flow through voltage-dependent channels. Altered calcium channels may be opened by less voltage comparing to wild-type channels which means that less depolarization is required to open a channel.11 Moreover, altered channels are opened for a longer time compared to wild type. Mutation Ser218Leu results in the development of the most altered channels and shows the most severe phenotype with risk of severe attacks accompanied by consciousness failures.17
Purpose of work - conduct analysis of molecular processes underlying pathogenesis of familial hemiplegic migraine type I. Building schemes of molecular interactions which lead to migraine attack development will allow not only to understand causes of an attack but also to reveal key molecules and pathways which may be targets for new drugs and treatment approaches.
In our study, we used Pathway Studio® 9 software with ResNet® 13 (Elsevier) database. ResNet13 contains biological objects (proteins, cell processes and diseases in particular) with annotations, as well as annotations of function links between objects. This database is a result of processing full-text research papers and abstracts indexed by Medline.
We created a hypothetical scheme of signal pathways describing causes and possible mechanisms of aura, vasodilation and pain in FHM1. The scheme is shown on figure 1, CACNA1A is marked white (Figure 1). Cav2.1 channel consists of four proteins CACNG2, CACNA2D2, CACNB4 and CACNA1A. Cav2.1 main function is modulation of dominantly excitatory neurotransmitter release in neuromuscular synapses as well as in central synapses of cerebellum, trunk and cortex.18
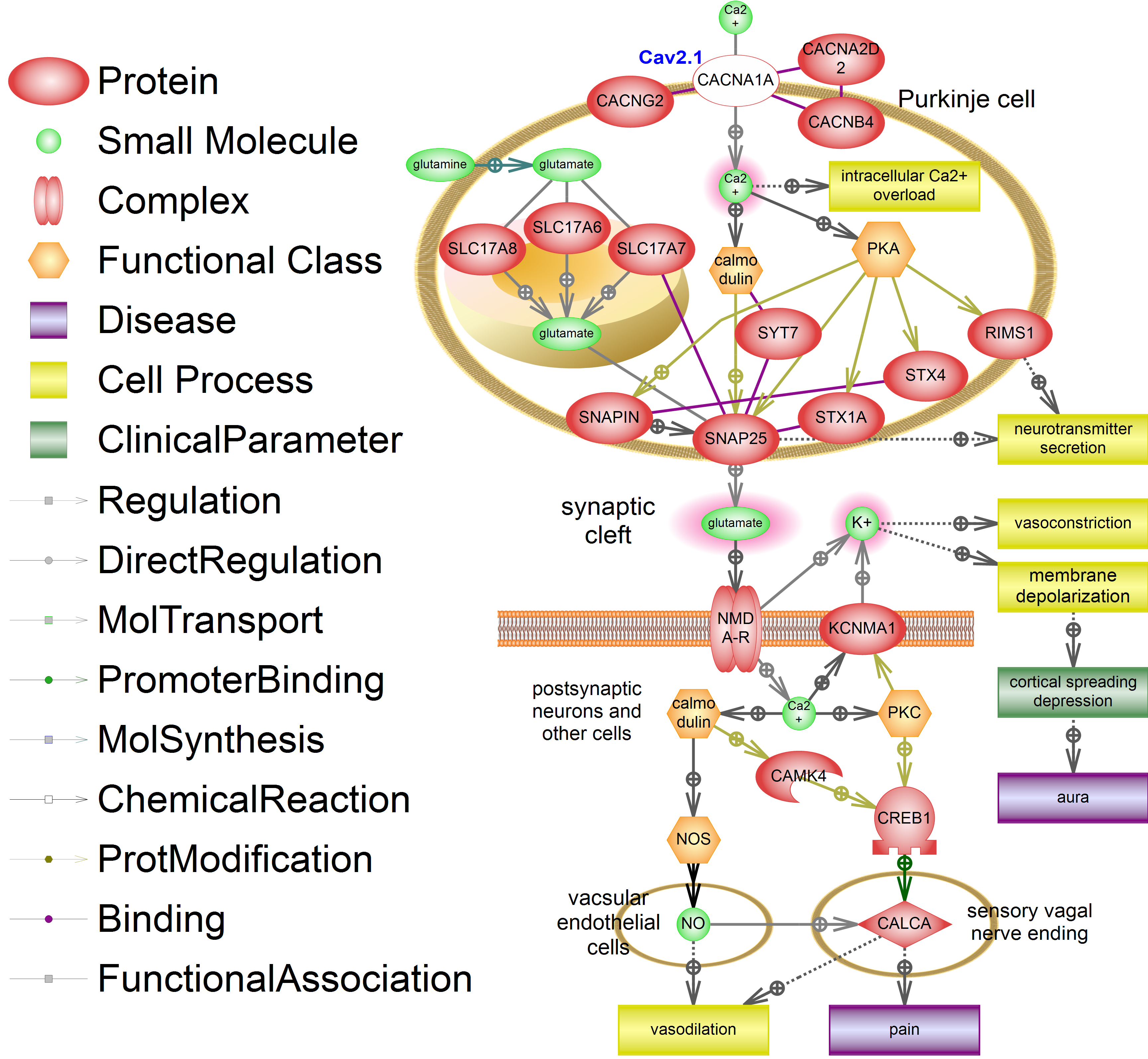
Figure 1 Signaling pathway of familial hemiplegic migraine type I (FHM1). Mutations in CACNA1A (white-out style) lead to intracellular calcium overload, glutamate overdose in synaptic cleft, development of spreading cortical depression (SCD), migraine aura, vasodilation and pain. Molecules with increased concentrations are highlighted in red. Legend is shown on the Figure. Designed in the Pathway Studio 9 ® (Elsevier). This signaling pathway is built manually using ResNet13 database ® (Elsevier).
In Purkinje cell 3 vesicular glutamate transporters - SLC17A7, SLC17A6 and SLC17A8 – transport synthetized glutamate to vesicles. Glutamine is being synthetized in astrocytes and transported to neurons. The fusion of vesicles with presynaptic membrane is initiated by intercellular calcium by activation of calmodulin and protein kinase A (PKA). Calmodulin and PKA phosphorylate activate main proteins of exocytosis such as SNAP25, SNAPIN, SYT7, STX1A, STX4 and RIMS1, which take part in neurotransmitter secretion.
Thus, CACNA1A dysfunction leads to uncontrollable calcium intake which leads to recurrent glutamate release to the synaptic cleft. This is supported by clinical data since glutamate release inhibitor (botulin toxin A) alleviates the migraine symptoms. Main target of botulin toxin A is SNAP25 a protein which plays lead role in fusion of vesicles containing neuromediators with cell membrane. Thus, the pathological increase of glutamate concentration in synaptic cleft is a key part of our scheme.
Increased glutamate release activates NMDA receptors on postsynaptic neurons which lead to release of intracellular potassium to cell surface and calcium intake. Extracellular potassium causes depolarization of membrane. Hyperpolarization is the main cause of spreading cortical depression characterized by spreading polarization of brain cells. Aura antecedent to migraine attack is the result of spreading cortical depression. Recent studies showed that the increase of glutamate concentration plays a leading role in the development of spreading cortical depression supporting the hypothesis of cortical hyperexcitability in migraine.19
Potassium release leads to vasoconstriction of nearby vessels. Vasoconstriction appears before vasodilatation and occurs in parallel with development of spreading cortical depression. Intracellular calcium in postsynaptic neurons activates KCNMA1 channel, leading to lengthening of depolarization. Intracellular calcium activates cell specific (neuronal or vascular-endothelian) types of NO-synthases (NOS) via calmodulin which results in increased synthesis of nitric oxide (NO) - a strong vasodilator and CGRP (CALCA) release activator. Synthesis and release of the main vasodilator and pain neuromediator CGRP (CALCA) in sensory vagus nerve ending is also activated. This neuropeptide causes the main pathological processes appearing in migraine attack: vasodilation and pain.
Thus we are the first to create a model of signal pathways leading to FHM type I. The key molecular in this model is synaptic glutamate. And major processes are intracellular calcium overload in Purkinje cell and membrane depolarization after glutamate overdose. This model may be used as a starting point for identification of further pathways of “common” migraine.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of Interest Statement
No conflict.
Author’s contributions
Conception: Eugene Klimov, Olga Rudko, Julia Azimova, Gyusal Tabeeva
Literature Analyses: Elena Naumova, Julia Azimova, Alexey Sergeev, Kirill Skorobogatykh, Anna Soboleva, Zarema Kokaeva
Pathway Design: Eugene Klimov, Artemiy Tretiakov, Vladimir Sobolev
Manuscript Preparation: Eugene Klimov, Artemiy Tretiakov
Writing of the first draft: Eugene Klimov, Olga Rudko.
None.
None.
None.

©2017 Klimov, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.