Journal of
eISSN: 2373-6410


Mini Review Volume 2 Issue 1
Department of Electrodiagnostic, Frank Pais Hospital, Cuba
Correspondence: Aymee Hernandez, Department of Electrodiagnostic, Frank Pais Hospital, 51 Aveneue and 22, La Lisa, Havana, Cuba, Tel 537-2503130
Received: October 18, 2014 | Published: February 16, 2015
Citation: Hernandez A. Evoked potentials as neurophysiologic tools to evaluate stroke. J Neurol Stroke. 2015;2(1):13‒17. DOI: 10.15406/jnsk.2015.02.00046
Stroke is one of most common cause of death in development countries and the most common disabling neurologic disorder. Diagnosis of the disease is base in clinical exam, history of patient and image techniques like computerized tomography scan (CT scan) and magnetic resonance images (MRI). Evoked potentials, usually not employed in diagnosis of stroke, are very useful in the evaluation of the patient functions and disabilities. For this reason we have a brief review of the utility of evoked potentials in the evaluation of the stroke in acute or subacute phase. Visual evoked potential modality evaluates the visual way, principally at optic nerve and optic chiasm level, or abnormalities of occipital areas. In patients with stroke that affect occipital cortex it usually show enlargements of P100 wave latency or amplitude diminish if there is axonal lesion of the nerve fibers. Brainstem evoked potential is useful to evaluate stroke when it occurs in brain structures that are irrigated for vertebrobasilar system. Somatosensory evoked potential show absence of N20/P25 complex or P40 response or amplitude diminishes of these responses; we can observe increase of the N13-N20 interval, always there is normality of the rest of the wave. Motor evoked potential parameters represent a useful early prognostic marker of motor function recovery in ischemic stroke patients. For other hand endogenous evoked potential such as: P300, N400, Mismatch negativity, Contingent negative variation and Premotor potential are very useful in the evaluation of cortical function in the stroke patients.
Keywords: Stroke, Visual Evoked Potential, Auditory Evoked Potential, Somato Sensory Evoked Potential, Endogenous Evoked Potential
CT Scan, Computerized Tomography Scan; MRI, Magnetic Resonance Images; ECG, Electrocardiogram; EEG, Electroencephalography; EP, Evoked Potentials; VEP, Visual Evoked Potential
Stroke is one of most common cause of death in development countries; it is the second cause of death in the world and the most common disabling neurologic disorder. Mundial incidence of this illness was estimated in 300 a 500/100.00 habitants per year for age between 45 and y 84 years old.1-3 Is a syndrome characterized by the acute onset of a neurologic deficit that persists for at least 24 hours, reflects focal involvement of the central nervous system and is the result of disturbance of the central circulation due to cerebrovascular disease.4-6 It has a lot predisposing factors and it can adopt varieties of clinical forms, patient evolution is variable; it depends of intensity, vessel and cerebral affected area.1,2
Diagnosis of the disease is based in clinical exam, history of patient and image techniques like computerized tomography scan (CT scan), magnetic resonance images (MRI) and other investigation methods are very important such as: some blood tests, electrocardiogram (ECG), Doppler ultra sonographic, echocardiography and Electroencephalography (EEG).7
Evoked potentials are usually not employed in diagnosis of stroke, but they are very useful in the evaluation of the patient’s functions and disabilities. This information is very important in the patient’s rehabilitation and rapid progression to daily activities.8 For this reason we have a brief review of the utility of evoked potentials in the evaluation of the stroke in acute or subacute phase.
Evoked Potentials (EP) are voltage variation that appears in cortical and subcortical structures of the nervous system in relation with an external stimulus or internal processing. They could be register by superficial electrodes in the skin or scalp by no invasive procedure.8,9
Clinical Value of Evoked Potentials
They evaluate sensory processing of the information in the nervous system. This technique has excellent temporal resolution and is easy to do. It is important to differentiate objectives and subjective events or when clinical exam is not possible in patients in a state of coma, encephalic death, psychiatric disorders, and children. It is a very useful technique in topographic localization of lesions or evaluating cognitive processing. The responses are very important to evaluate drug effects in some illness.8-11 As a descriptor, EP could be classified as exogenous (sensory) or endogenous (cognitive).
Exogenous or Sensory Evoked Potentials
They evaluate sensory processing of the information in the nervous system. This technique has excellent temporal resolution and is easy to do. It is important to differentiate objectives and subjective events or when clinical exam is not possible in patients in a state of coma, encephalic death, psychiatric disorders, and children. It is a very useful technique in topographic localization of lesions or evaluating cognitive processing. The responses are very important to evaluate drug effects in some illness.8-11 As a descriptor, EP could be classified as exogenous (sensory) or endogenous (cognitive).
Endogenous or Cognitive Evoked Potentials
They are not specific by modalities. They reflect the activity of the neural network in relation to the cognitive processing of sensory information. They are evoked by external or internal stimulus and could appear in absence of stimulus. They do not depend on the physical properties of stimulus. They are in temporal relation with the central processing incoming afferent signals. Latency of the response is large. They are sensitive to the subject state. It includes P300, N400 responses, Mismatch negativity, Contingent negative variation and Premotor potential.8-11
Visual Evoked Potential (VEP)
This evoked potential modality evaluates the visual way, principally at optic nerve and optic chiasm level or abnormalities of occipital areas. It is useful in vascular diseases that affect optic nerve or occipital areas.8-11 They are evoked by applying a visual stimulus (flash, pattern reversal or LED). Principal responses are N75, P100 and N135 responses. P100 response is generated at cortical occipital area.8-11 Stroke can cause abnormalities at nerve optic level or cortical blindness.4
Ischemic ocular syndrome is a disease caused by vascular disease at a level of common or internal carotid artery. It diminishes perfusion in retinal center artery, and can cause visual defect and nerve optic atrophy. In this case VEP is very useful because it study conduction of optic nerve fibers, which could be damage. Usually VEP show enlargements of P100 wave latency or amplitude diminish if there is axonal lesion of the nerve fibers. It has been reported by Chen et al. & Acosta et al.12,13
Rodriguez et al. & Stuss et al.14,15 and other authors described VEP abnormalities in stroke that affect visual cortex. In cortical blindness due to stroke VEP can show absence of all components in some cases, but in others can show normal P100 response, its occurs in patients who have retinogeniculate fibers and parts of cortical areas functional. This means that a surviving neuronal pool in area 17 generates a P100 potential, but is not sufficient for visual perception.12-16 On the other hand, Hodelin et al.16 reported utility of VEP in diagnosis of conscious disturbances, especially brain death.
Auditory Evoked Potential
The auditory modality with more clinical application is Brainstem Auditory Evoked Potentials (BAEP) (Figure 1). This technique studies auditory processing in brainstem structures.8-11 It is useful to evaluate stroke when it occurs in brain structures that are irrigated for vertebrobasilar system. In some cases its can occur silently.8,17-19 This technique consists of apply an auditory stimulus (click), which evokes five waves: I and II (generate at cochlear nerve), III wave (generate at cochlear nucleus of the protuberance), IV wave (generate at lateral lemniscus) and V wave (generate at inferior colliculus of the midbrain).8-11

Figure 1 Brainstem Auditory Evoked Potential in a case of patient who suffers irrigation deficits in vertebrobasilar system. He suffers from vertigo. Note increase of latency of III, IV and V waves. It is in relation of abnormalities of conduction time al level of auditory way at brainstem structures.
Abnormalities of BAEP in stroke: If midbrain structures are affected we can observe diminished amplitude of V wave or absence of IV and V waves. If protuberance structures are involved we can see absence of III, IV and V waves or III-V and I-V intervals prolonged. When brain death is suspected BAEP can show absence of all waves, except I wave, which is normal bilaterally.17-21 Lee, Bovo and Alpini18-20 have reported the utility of BAEP in diagnosis of vascular disease that affected brain stem; they empathized in the diagnosis and prognosis value of this technique.17-21
Somatosensory Evoked Potentials (SEP)
This modality is evoked applying an electrical stimulus to the skin surface. It is a response that is evoked by electrical stimulation of nerve trunk of upper or lower extremities.8
If nerves of upper extremities are stimulated some wave forms appear (Figure 2):

Figure 2 Somatosensory Evoked Potential of left median nerve in a case of patient with right parietal infarction. Note increase of latency of N20 and P25 complex with normality of the other waves.
If nerves of lower extremities are stimulated more common wave forms that appear (Figure 3):
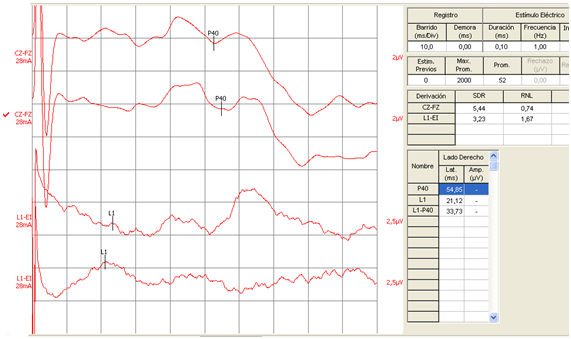
Figure 3 Somatosensory Evoked Potential of right posterior tibial nerve in a case of patient with right parietal infarction. Note increase of latency of P40 wave with normality of lumbar wave.
Abnormalities of SSEP in stroke:
They are useful evaluating cortical or brainstem vascular diseases.4 In case of parietal or thalamic lesions, SSEP show some abnormalities as absence of N20/P25 complex, absence of P40 response or amplitude diminishes of these responses. We can observe increase of the N13-N20 interval too. Always there is normality of the rest of waves.8-11 Experimental studies have shown that recovery was better when early SSEP remained unaffected during middle cerebral artery occlusion.22-25 Several investigations have reported better functional prognosis in stroke patients with persistence or early recovery of SSEP.22-25
Other studies have shown that the recovery of SSEP may be incomplete long after the disappearance of the CT scan hyper density in patients with small capsular hematoma.22-25 Although SSEP does not replace the clinical examination, it can provide a more objective measure of sensory pathways and is particularly useful in patients with dysphasia, inattention or decreased level of consciousness as well as a good predictor of outcome.24 Al-Rawi et al.25 demonstrated that the SSEPs N20 latency can predict, even roughly the size of cerebral infarction, whether lacunar or large-vessel stroke.25
Motor Evoked Potential (EP)
It is a response evoked by electrical or magnetic stimulation of corticospinal tract at cortex or spinal level. It is registered at upper or lower extremity muscle.8-11 Escudero et al.26 demonstrated that amplitude of MEP is the most sensitive parameter of predictive value in stroke out coming. (Different font selection Cambria versus Calibri)They supported the idea that MEP obtained by transcranial magnetic stimulation represents a useful early prognostic marker of motor function recovery in ischemic stroke patients. In addition, the technique could be used for monitoring and quantifying motor function, in parallel with corticospinal tract permeability, in the course of patient recovery. On the other hand, Heald et al.27,28 in 1993 concluded that the presence of MEP with normal or delayed CCT is able to differentiate a group of patients with a high probability of survival and good functional recovery. In contrast, its absence would indicate poor recovery or an increased risk of death.
Rapisarda et al.29 in 1996 performed a TMS study of 26 acute ischemic stroke patients, with a follow-up of 14 days. These patients had a brain infarct in the sylvian region, with total hand paralysis. MEPs were present in 42.6% of patients, and their presence with an amplitude >5% that of the amplitude of the M wave implied a favorable prognosis for motor function recovery. According to these authors, MEP amplitude was of greater value than MEP latency. However, patient follow-up was very short, and although a large number of stroke patients recovered motor function, mainly during the first month, some patients had slower or later recoveries.
In contrast to these studies, in support of the prognostic value of the technique, other authors failed to corroborate its utility, such as: Rijckevorsel-Hartman et al., Zgur et al. & Arac et al.26 Endogenous evoked potentials are very useful to evaluate cognitive function in patients who suffers vascular disease. In clinical practice the most useful potentials are: P300, N400, Mismatch negativity, Premotor potential, and Negativity contingent variation.8
P300 Potential
Is a response to unexpected visual, auditory or tactile stimulus? It´s appearance is associated to oddball paradigm, which consists in presentations of sequences of repetitive audio/visual stimuli are infrequently interrupted by a deviant stimulus. The subject is asked to react either by counting or by button pressing incidents of target stimuli that are hidden as rare occurrences amongst a series of more common stimuli, which often require no response.
It is a positive wave form that appears across the parieto-central area of the skull, is maximal at the vertex and it is usually around 300ms. In their genesis are involvements some structures like: temporo-parietal junction, motor supplementary cortex, cingulated anterior cortex, upper temporal gyrus, insula, prefrontal and dorsolateral cortex, hippocampus, and amygdale.30
Abnormalities of P300 potential in stroke: Latency enlargement, amplitude diminishes of global form. In some cases there is absence of response, fundamentally at frontal areas.31-33 Several authors such as De Quesada have reported that some asymptomatic hypertensive patients could show all of these abnormalities of P300 wave. It could demonstrate that these patients could have silent cerebral lesions.30
N400
Is a negativity that appears at 400 millisecond in relation with a task of semantic processing. This wave is evoked by a word or phrase that is semantically incongruent. It is originated at medial temporal gyrus of the dominant hemisphere.34-36
Abnormalities of N400 in stroke: Grieder et al.37 has reported abnormalities of N400 waves in relation with diminished cerebral blood flow in the anterior temporal lobes. They had suggested that N400 topography alterations might be a potential marker for the detection of early dementia, primarily vascular dementia.37-42
Mismatch negativity: This wave reflects presentational capacity of detection of auditory stimulus change. It is originated in primary auditory cortex.43-45 In stroke that involvement temporal lobes, Mismatch negativity shows amplitude diminish. It has great importance in auditory rehabilitation of the aphasias and it is an important predictor of functional recuperation in Coma.46,47
Contingent Negative Variation (CNV)
It is maximal at the vertex; it represents the mental preparation to response to stimulus, It´s a negative wave form. It is generated during wait period to do a task. It is very useful in the evaluation of pre-frontal cortex function.48-50
Premotor potential or Bereitschafts potential: It precedes a voluntary movement. It is generated during wait period to do a motor task, it is maximal at vertex, it is a negative wave form. It is a sign of general preparation for premeditated voluntary acts and it is directly related only to the initiation and motor control of movement. It is very useful in the evaluation of motor pre-frontal cortex function.51-53
Evoked potentials are not used like routine diagnostic methods in diagnosing stroke, but they are very useful in diagnosis of complication, prediction of evolution and disabilities of the stroke. They are being studied in investigative field.
None.
None.

©2015 Hernandez. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.