Journal of
eISSN: 2373-6410


Case Report Volume 12 Issue 6
1Department of Interventional Neuroradiology, Federal Fluminense University, Brazil
2Department of Interventional Neuroradiology, Niterói D’Or Hospital, Brazil
3Department of Neurosurgey, Federal Fluminense University, Brazil
Correspondence: Guilherme de Palma Abrão, Departamento de Radiologia do Hospital Universitário Antonio Pedro, R Marquês de Paraná,303- Centro, Niteroi- RJ, Brazil, 24033-900
Received: September 29, 2022 | Published: November 1, 2022
Citation: Abrão GP, Barbosa MM, Marques MCP, et al. Endovascular treatment of anterior communicating artery ruptured blister-like aneurysm with flow- diverter stent. J Neurol Stroke. 2022;12(6):172-174. DOI: 10.15406/jnsk.2022.12.00521
Blister-like aneurysms (BLAs) are rare lesions, described as a small sac with thin wall and broad-based neck. Although its typical location is the dorsal wall of the supraclinoid internal carotid artery (ICA) at non-branching sites, atypical locations as the anterior cerebral artery (ACA) are reported. Due to its fragile walls, BLAs are associated with diffuse subarachnoid hemorrhage (SAH) and tend to rebleed, thus requiring emergency treatment. Endovascular and surgical approaches in acute setting are both considered challenging due to aneurysm morphological features. Here we describe a case of a ruptured blister-like aneurysm of the anterior communicating artery treated by endovascular exclusion of the Anterior Communicating Artery (AComA) using micro-coils and deployment of flow diverter stent (FDS) in the left anterior cerebral artery. Control angiogram confirmed exclusion of the lesion and patency of the left ACA. Patient presented no new neurological deficits or procedure complications. We suggest that in selected cases, FDS treatment for blood blister-like aneurysm in atypical locations can be safe and effective when no other treatment is available.
Keywords: blister-like aneurysm, flow-diverter stent, subarachnoid hemorrhage
BLA, blister-like aneurysm; SAH, subarachnoid hemorrhage; AComA, anterior communicating artery; FDS, flow-diverter stents; MCA, middle cerebral artery; ACA, anterior cerebral artery; BA, basilar artery; DSA, digital subtraction angiography
Blister-like aneurysms (BLAs) are considered rare lesions, described as a small sac with thin wall and broad-based neck. Although its typical location is the dorsal wall of the supraclinoid internal carotid artery (ICA) at non-branching sites, atypical locations reported comprise anterior cerebral artery (ACA), middle cerebral artery (MCA), and basilar artery (BA).1 Anterior Communicating Artery (AComA) is the most common atypical site.2 This lesion is more frequently observed in females, at younger ages, and is associated with hypertension. BLAs are also associated with diffuse subarachnoid hemorrhage (SAH) and tend to rebleed3 owing to its thin and fragile walls, thus requiring emergency treatment. However, current imaging exams lack sensitivity and a group of patients may need repeated digital subtracted angiography (DSA) to highlight diagnosis.1,4 There is no consensus about the treatment of choice and decision should be made case by case. Endovascular and surgical approaches in acute setting are both considered challenging due to aneurysm wall fragility and its small sac and strategies should be discussed in a multidisciplinary team. Surgical techniques include parent vessel occlusion, clipping and wrapping-clipping. Endovascular strategies consist of parent vessel occlusion, coiling, stent assisted coiling and flow diversion.2–4
A 35-year-old previously healthy woman presented in emergency department complaining of severe headache, with a Grade 2 Hunt and Hess score. Neurological status was unremarkable. A computed tomography (CT) scan showed SAH (Modified Fisher Scale grade I) and the cerebrospinal fluid (CSF) sample showed 12.000 RBC/CC. Nevertheless, CT angiography did not depict any saccular aneurysm. Due to high suspicion, a three-dimensional rotational angiography was performed on day two, but showed only some minor AComA wall irregularities close to the left A1/A2 junction (Figure 1A). A second angiogram was performed 6 days later in order to elucidate the cause of the SAH and revealed a small pseudoaneurysm at this site (Figure 1B). Considering the acute morphological changes and the previous blending, we indicated urgent treatment. Microsurgical clipping occlusion of the AComA was discarded as an option since the lesion stretched out to the left A1/A2 segment. Since right A2 segment was supplied by right A1 segment, we decided to occlude AComA and the pseudoaneurysm with coiling and perform endovascular reconstruction of the left A1/A2 segments with a Flow Diverter Stent (FDS). The procedure was performed under general anesthesia and systemic heparinization (8000 IU during the procedure). The AComA occlusion was achieved with remodeling technique. A Scepter XC balloon 4 x 11m (Microvention Endovascular Inc., CA, USA) protected the left A1/A2 junction and three Microplex coils (Microvention Endovascular Inc., CA, USA) were detached through a Headway 17 microcatheter (Microvention Endovascular Inc., CA, USA). Finally, 2,5 x 14 x 20 mm Fred Jr Embolization Device (Microvention Endovascular Inc., CA, USA) (Figure 2) was placed through a Headway 21 microcatheter (Microvention Endovascular Inc., CA, USA), covering all the A1/A2 junction. Immediately after its placement, we administered intravenous abciximab 0.25 mg/kg bolus and maintenance dose for 12 hours. Dual antiplatelet therapy was initiated in the following day with clopidogrel 75 mg and aspirin 100 mg and kept for three months while aspirin was sustained for one year. External ventricular derivation was not required since there was no ventriculomegaly. Patient presented no evidence of new neurological deficits or complications such as rebleeding, regrowth, vasospasm, stent occlusion/stenosis or stroke. Control angiogram performed three months later confirmed exclusion of both pseudoaneurysm and the AComA (Figures 3) without any persistent arterial wall irregularity. Patient remained clinically stable with mRs 0.
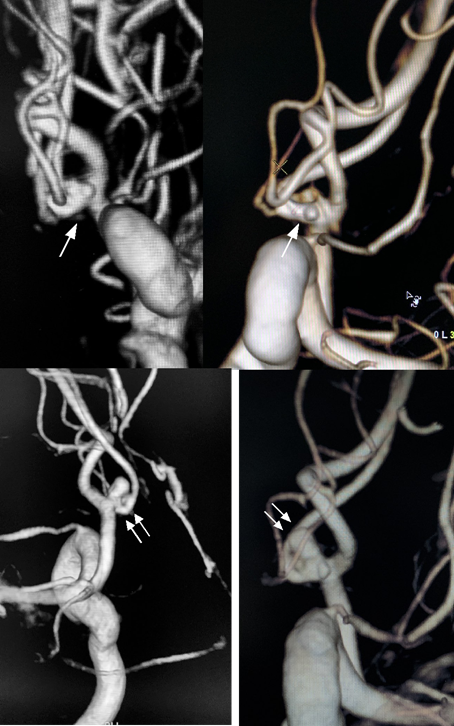
Figure 1 On day one of hospitalization 3D angiography showed some minor irregularity of the AComA close to the left A1/A2 junction (arrow) (A and B). At day 7, 3D angiography revealed an pseudoaneurysm on the AComA (double arrow) with arterial wall irregularities on the AComA and on the left A1/A2 junction (C and D)..
The complexity of this condition is reflected on published data and it’s pathophysiology still poorly understood. Histopathological analysis indicate that BLAs are not true aneurysms, as they lack intima and media, which results in wall fragility in a focal arterial segment. This characteristic suggest that they have acute dissecting etiology, which differentiates this group from typical saccular aneurysms.2,3 In morphological aspect they exhibit a poorly defined broad-based neck with a small aspect ratio and resemble a blood blister.1,2,3 Due to this combination, ruptured BLAs are hazardous and tend to regrowth and rebleed. The presence of acute configurational changes, as occurred in our case, makes treatment planning even more challenging and highlights the urgency of treating the lesion. According to Peschillo et al.,2 although they typically occurs at the dorsal wall of supraclinoid internal carotid artery (ICA) commonly at non-branching sites about 8.6% of the BLAs occur in atypical sites and the most frequent atypical site is the location we report here, the AComA. A systematic literature review conducted by Gonzales et al.4 indicates that BLAs are more frequently observed in females, at younger ages, and is associated with hypertension. These rare lesions remains a treatment and diagnosis challenge, as previously reported by Andaluz et al.,1 since, because of its small size, imaging exams, particularly CT angiography, lack sensitivity. Thus, catheter cerebral angiogram is nowadays a paramount exam, considered the gold standard in the context of SAH and negative CT angiogram findings. The most common clinical presentation of BLAs is SAH and some authors reported a 99% incidence.4,5 Still, BLAs might comprise one of the causes of angiogram-negative SAH, particularly when the distribution of blood on CT is non-perimesencehpalic.6 They are prone to dynamic changes in size and morphology and rates of regrowth and rebleed reach 15% and 23% respectively.4 For this reason, prompt diagnosis and treatment are mandatory. Therefore, repeated angiographies may be necessary to elucidate diagnosis.3 Besides its sensibility, 3D digital subtraction angiography (DSA) allowed us the observation of acute morphological changes in the context of minor irregularities and permited accurate measures which helped on treatment decisions. Our patient was initially reported as having a negative CT angiogram and a minor abnormality in the AComA on DSA. One week after, a 3D angiogram showed a pseudoaneurysm in AComA and irregularities in the left A1-A2 segment, and patient was submitted to urgent treatment. Concerning the treatment, there is no consensus about the optimal option and decision should be made case by case. Surgical techniques include parent vessel occlusion, clipping and wrapping-clipping. While endovascular strategies consist of parent vessel occlusion, coiling, stent assisted coiling and flow diversion.4,5,7,8 Small size and wall fragility disfavors isolated clipping or coiling. Wrapping is associated with occurrence of rebleeding. Parent vessel occlusion remains the simplest treatment, using either the endovascular or surgical approach when possible.1 However, it could not be our only strategy since there was a minor irregularity in A1/A2 segment and at this point this circulation is considered terminal, what could result in ischemic injury. Rouchaud et al,9 described a case of BLA in the AComA treated by endovascular exclusion of the artery using two FDS in which initial 3D digital angiogram reveled very small arterial wall irregularities (<1 mm) without a well-defined neck and these findings were considered to be BLA. Recently, the concept of endoluminal arterial reconstruction is becoming widespread and emerge as a option of a more physiological method, who maintains the patency of the vessel. FDS are being increasingly used to treat these aneurysms, with several clinical series supporting its use.4,8,9 A observational retrospective study showed some tendency for lower morbi-mortality rates and more favorable outcomes (mRs < 3) in endovascular group.7 Zhu et al.8 estimated occlusion rates in treatment with FDS of 79% with 13% of recurrence and 3% of rebleeding and mortality. Published data suggest that mid to longterm aneurysm occlusion rates are better and the need for retreatment is low with FDS,3 but its efficacy still needs larger series for validation. The two major disadvantages of FDS are the need of dual antiplatelet in the context of acute SAH and low initial occlusion rates.5 In our case, we decided to occlude the pseudoaneurysm with micro-coils due to its rapid growth and to performendoluminal reconstruction of the left A1/ A2 segments excluding AComA using a FDS, as we could not identify for sure which one of the irregularities corresponded to the bleeding lesion. Antiplatelet therapy must be cautious in patients with SAH due to the potential need for neurosurgical procedures in those subjects (i.e. craniotomy, ventricular catheter placement). Thus, it is recommended to perform ventriculostomy, if needed, before starting dual antiplatelet therapy for the purpose of avoiding hemorrhagic complications.1,9 However, this management is still controversial in the setting of acute SAH and one should carefully evaluate the risk / benefit profile of potential hemorrhagic and thromboembolic complications in deciding on such therapy.10
BLAs are rare lesions and its pathophysiology is still poorly understood. Their wall lack intima and media and arterial dissection seems to be a possible mechanism of formation. Because of this characteristics, BLAs tends to regrowth and rebleed and urgent treatment is mandatory. Both diagnostic and treatment can be challenging. In cases with high suspicion, it is important to repeat DSA in order to identify the lesion. There is no consensus about the best treatment strategy, but FDS are being increasingly used and published data suggested that they can be safe and effective in the context of ruptured BLAs.
None.
The authors declare that there are no conflicts of interests.

©2022 Abrão, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.