Journal of
eISSN: 2373-6410


Research Article Volume 13 Issue 6
Department of Neurosciences, East Avenue Medical Center, Philippines
Correspondence: Maria Kim C Hernandez MD, East Avenue Medical Center, G/F Department of Neurosciences, East Avenue Medical Center, Quezon City, Metro Manila, Philippines
Received: October 17, 2023 | Published: November 2, 2023
Citation: Hernandez MKC, Joyce Angelie ST, Juangco DNA. Efficacy of remote ischemic conditioning in acute ischemic stroke patients receiving thrombolysis and thrombectomy: a meta-analysis. J Neurol Stroke. 2023;13(6):152-156. DOI: 10.15406/jnsk.2023.13.00564
Introduction: Remote ischemic conditioning is a non-invasive, easy-to-administer procedure providing brief, reversible episodes of ischemia conferring global protection to remote tissue or organs. In this study, we performed a meta-analysis on the available studies on patients receiving thrombolysis and thrombectomy.
Methods: PubMed, Cochrane, Embase, and ClinicalTrials.gov were searched for articles from inception to 10 September 2023. Data were analyzed using Cochrane RevMan Web. Odds ratio (OR), mean difference (MD), and 95% confidence intervals (CI) were combined via fixed-effect analysis.
Results: Seven randomized controlled trials were included with a total of 927 patients. Remote ischemic conditioning could reduce the recurrence of ischemic stroke at endpoints (OR 0.84, [0.30, 2.29]) and improve the clinical outcome (modified Rankin Scale 0-2) at 90 days (OR 0.96 [0.67, 1.36]) but the results are not significantly different from the control group.
Conclusions: Remote ischemic conditioning shows promise in reducing ischemic stroke recurrence and improving patients’ prognosis at 90 days.
Keywords: acute ischemic stroke, remote ischemic conditioning, thrombolysis, thrombectomy, meta-analysis
Cerebrovascular disease and cardiovascular disease are the most common cause of mortality in the world.1 In 2020, stroke accounted for 1:21 deaths in the United States alone.1 It is even projected to increase to 89.32 per 100,000 population by 2030.2 Stroke can be ischemic or hemorrhagic. Around 87% of all strokes are ischemic while the remaining are hemorrhagic.3 The main treatment for ischemic stroke is the administration of intravenous alteplase, which must be given within 3 hours and up to 4.5h in selected patients.4 However, rapid reperfusion after a period of ischemia may induce ischemia-reperfusion injury (IRI).5 This is common in settings of thrombolysis such as in acute ischemic stroke or myocardial infarction.5
Remote ischemic conditioning (RIC) introduces brief, reversible episodes of ischemia and reperfusion applied in one vascular bed, tissue, or organ gives global protection rendering remote tissues and organs resistant to ischemia or reperfusion injury (Figure 1).6 In RIC, studies have employed 3 or 4 cycles of 5-min arm or leg ischemia followed by 5-min reperfusion periods.6 These are empiric and optimal dose is not yet established.6 Three methods of RIC have been reported. These are remote ischemic preconditioning (RIPreC, given before IRI), remote ischemic preconditioning (RIPerC, given after the onset of ischemia), and remote ischemic postconditioning (RIPostC, given at the reperfusion stage).5 The mechanisms underlying RIC have not been fully explored but it includes neuronal and humoral pathways as well as the modulation of immune-inflammatory responses.5
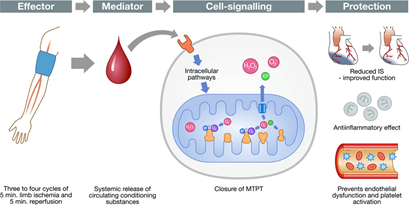
Figure 1 Pathophysiology of remote ischemic conditioning. Image lifted from Schmidt et al.19
This study provides evidence of neuroprotection of RIC in acute ischemic stroke (AIS) patients who underwent thrombolysis and mechanical thrombectomy.
A meta-analysis was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Search strategy and study selection
We performed a systematic search of the Pubmed, Cochrane, Embase, and ClinicalTrials.gov databases from inception to 10 September 2023 comparing the efficacy of RIC and sham-RIC in AIS patients. Database specific search terms for RIC and AIS were combined by limiting the searches to studies of human patients and reports of clinical trials. Search terms used were (acute ischemic stroke) AND (remote ischemic conditioning) AND (randomized controlled trial). Reference lists of eligible studies were hand-searched to avoid missing any relevant studies. Two reviewers independently assessed the eligibility of potential studies. They also independently extracted date, assessed risk of bias and summarized strength of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. Methodological approach included the selection criteria development, search strategies definition, quality assessment of eligible studies, data extraction, and statistical analysis. We screened 350 articles and assessed 17 articles for eligibility. Seven studies were included in the quantitative synthesis. See Figure 1 for the summary.
Inclusion and exclusion criteria
Inclusion criteria for this study were (1) randomized controlled trials (RCTs); (2) adults (≥18 years old) diagnosed with AIS; (3) underwent thrombolysis and/or thrombectomy; (4) RIC intervention consisted of RIPreC, RIPerC, or RIPostC; and (5) the control group either receiving sham RIC, which required application of an occlusive device without complete blood flow occlusion) or without sham procedure.
Exclusion criteria were (1) study designs other than RCT, (2) RCTs without available results, (3) animal interventions, (4) articles written in language other than English, (5) studies without outcomes of the present research, and (6) studies with cognition and post-stroke complication outcomes.
Data extraction
Two reviewers independently extracted the following data: (1) article identification (authors, country of publication, year published, journal publication, total number of participants, intervention received, patient characteristics) and (2) outcome measures.
Assessment of methodological quality
Quality of each study was assessed based on the Cochrane Handbook for Systematic Reviews of Interventions. Each of the items was scored as low risk, unclear risk, or high risk.
Statistical analysis
Statistical analyses were performed using Cochrane RevMan Web. Risk difference for dichotomous data were estimated for the effect size. Confidence intervals (CI) at 95% are presented. Fixed effect model is shown herein as well. In addition, heterogeneity between studies was examined via the I2 statistic.
A total of 350 studies were identified and screened. Only 17 studies were assessed for eligibility and 7 studies were included in the quantitative synthesis (Figure 2). Primary outcome was extracted from each trial. Main characteristics of the trials are shown in Table 1. The quality assessments of each trial are summarized in Table 1. Randomization was done in all seven RCTs. Fifty-seven percent for the RCTs did not provide allocation concealment information. All of the included studies were blinded to observers. Four RCTs provided drop-out description.
|
Authors |
Country |
Year |
Journal |
Total number of participants |
Standard acute ischemic stroke treatment |
Type of remote ischemic conditioning |
Number of patients with Remote Ischemic Conditioning |
No. of patients with sham Remote Ischemic Conditioning /control |
Outcomes |
|
An et al.8 |
China |
2020 |
Neurology |
68 |
rt-PA |
RIPostC |
34 |
34 |
c, e, g |
|
Che et al.9 |
China |
2019 |
Annals of Clinical and Translational Neurology |
30 |
rt-PA |
RIPostC |
15 |
15 |
a, b, f, |
|
England et al.10 |
United Kingdom |
2019 |
Journal of the American Heart Association |
60 |
rt-PA |
RIPerC |
31 |
29 |
a, b, c, d, g |
|
He et al.11 |
China |
2020 |
Annals of Clinical and Translational Neurology |
49 |
rt-PA |
RIPerC |
24 |
25 |
a, b, c, d, g |
|
Hougaard et al.12 |
Denmark |
2013 |
Stroke |
453 |
rt-PA |
RIPerC |
247 |
196 |
c, d, g |
|
Landman et al.13 |
Netherlands |
2022 |
International Journal of Stroke |
88 |
rt-PA and thrombectomy |
RIPostC |
40 |
30 |
b, c, d, g |
|
Pico et al.14 |
France |
2020 |
JAMA Neurology |
188 |
rt-PA and thrombectomy |
RIPerC |
93 |
95 |
b, c, d, f, g |
Table 1 Summary of studies included in the meta-analysis
Note: a – recurrence of ischemic stroke (IS) at endpoint, b – endpoint NIHSS score, c – modified Rankin Scale (mRS) 0-2 at 90 days, d – dependency (mRS 3-5) at 90 days, f – RIC-related adverse events, g – death
Outcomes
Recurrence of ischemic stroke (IS) at the endpoint: Three RCTs stated IS recurrence at the endpoint as a primary outcome. One hundred thirty-nine patients were pooled from 3 RCTs. Although the results are not significant, the forest plot (Figure 3) showed that RIC could reduce the recurrence of IS at the endpoint (OR 0.84, 95% CI [0.30, 2.29].
Modified Rankin Scale 0-2 at 90 days: Six out of 7 RCTs specified an mRS 0-2 at 90 days as one of the endpoints (OR 0.96 [0.67, 1.36]). Subgroup analysis was done between the number of limbs occluded (Figure 4). The unilateral limb occlusion subgroup favored the sham RIC while the bilateral limb subgroup favored RIC.
Modified Rankin Scale 3-5 at 90 days: Dependency at 90 days was also explored in 6 out of 7 RCTs as seen in Figure 5. Overall, RIC reduced dependency of patients at 90 days (OR 0.91 [0.62, 1.33]). Subgroup analyses were done between the number of limbs occluded and results were not significantly different.
Risk of bias assessment and quality of evidence: Figure 6 summarizes the risk of bias in the included studies. In all RCTS, patients were randomized into either intervention or control group. Three RCTs had unclear risk on allocation concealment.10,13,14 No information was provided in these RCTs. High risk performance biases were identified with four studies.8,9,11,12
Publication bias: Figure 7 shows the funnel plot for the studies included in this meta-analysis. Publication bias was less likely in the included studies.
Upper plot: studies with recurrence of ischemic stroke at the endpoints, middle plot: studies with an mRs 0-2 at day 90, bottom plot: studies with an mRS 3-5 at day 90. There were some limitations in our meta-analysis. Studies with patients undergoing thrombolysis and thrombectomy were included in this review. Also, the studies which included thrombectomy did not indicate outcome of patients who were randomized to either RIC or sham RIC/control. The sample sizes of the two studies were larger than the rest of the study, which could potentially pose bias to the results. Also, subgroup analysis for the number of cycles and cycle duration was not explored.
Thrombolysis remains the gold standard in the treatment of acute ischemic stroke.14 Recent studies on mechanical thrombectomy showed improvement in clinical outcomes versus standard care in select patients.15 Several adjunct treatments have been studied but the most promising drugs include oxidative and nitrosative stress inhibitors and promotors of neurogenesis/ -regeneration/ and -recovery.16 RIC is an easy-to-use, non-invasive therapy, and has been well-tolerated in patients with AIS and subarachnoid hemorrhage.17 RIC provides systemic protection by inducing a brief period of focal ischemia followed by reperfusion.17 It confers protection against severe ischemia in distant organs.17 Mechanisms are not fully understood but it has been shown to increase cerebral tolerance to ischemic injury, cerebral infarction reduction, improvement in cerebral perfusion, and promote cerebral collaterals formation.17
This study sought the effects of RIC on AIS patients. A total of 7 studies with 927 patients were included. Our study showed that the addition of RIC to standard IS treatment has no significant statistical difference. However, it is worth noting that RIC decreased the recurrence of IS and improved clinical outcomes at 90 days. Only three studies explored the recurrence of IS as an outcome. Based on Figure 2, patients treated with RIC decreased the recurrence of ischemic stroke. This was the same in a previous study done by Kan et al. in 2023. RIC generally showed advantages compared with sham RIC/control in improving the prognosis and dependency of a patient as seen in Figures 3 and 4. RCTs shown are not statistically significant from each other. In the subgroup analysis done for the number of limbs occluded, results were also not significantly different but were heterogenous. Bilateral limb occlusion might be more beneficial in improving the outcomes at 90 days. Kan et al. (2023) stated that bilateral limb occlusion for 5 cycles at a length of 50 mins might be an optimal protocol for AIS patients. This may be due to the increased neuronal, humoral, and immunomodulatory factors involved as compared to occluding a unilateral limb alone. As seen in Figure 4, majority of studies showed improvement of based on the mRS with the RIC group (OR 0.91 [0.62, 1.33]). No heterogeneity was observed.
In summary, there was no statistically significant difference in the addition of RIC in AIS patients. However, it showed trends reduce the recurrence of IS at the endpoint and improve patients’ clinical outcomes at 90 days. There are still no optimal or standard cycles of RIC. It is a promising adjunct treatment and a consensus may be done in the future while awaiting standardization of treatment.
None.
The authors have declared no conflict of interest.

©2023 Hernandez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.