Journal of
eISSN: 2373-6410


Research Article Volume 4 Issue 2
1Assistant lecturer of Neurology, Alexandria University, Egypt
2Lecturer of neurosurgery, Alexandria University, Egypt
3Assistant Professor of Neurosurgery, Alexandria University, Egypt
4Lecturer of neurosurgery, Alexandria University, Egypt
5Consultant of neurosurgery, Tawam Hospital, UAE
6Professor of Neurosurgery, Alexandria University, Egypt
7Lecturer of neurosurgery, Alexandria University, Egypt
Correspondence: Aziz Waseem, Lecturer of neurosurgery, Alexandria University, Chamblion Street, Azarita district, postal code: 21531, Egypt
Received: October 06, 2014 | Published: January 20, 2016
Citation: Mohamed A, Ahmed S, Tamer H, Tamer I, Amr ElS, et al. (2016) Cranial Arteriovenous Malformations During Pregnancy: A Multidisciplinary Algorithm for Safe Management. Case Series and Review of the Literature. J Neurol Stroke 4(2): 00122. DOI: 10.15406/jnsk.2016.04.00122
Background: Cerebral arteriovenous malformations (AVMs) are relatively uncommon lesions in general population and rare among pregnant females, meanwhile they carry a relatively higher morbidity and mortality. In view of limited data for optimal management, we try to address dilemmas commonly encountered in pregnant females with cranial AVM and propose multidisciplinary algorithms for safe management.
Methods: Five cases of cerebral AVM pregnant females (ruptured during pregnancy) were followed up for 2 years after diagnosis. Endovascular embolization was performed in 3 cases and the other cases underwent expectant management. Natural history regarding rebleeding, time at bleeding, pregnancy and obstetric management as well as the endovascular management and teratogenicity issues were the primary outcome measures. Besides, we conducted a review of literature (Pubmed, Sciencedirect and other databases) during the years 1972 to 2013.
Results: We categorized the obtained data into key issues. The effect of pregnancy as a risk for AVM rupture appeared to be ill defined. The best time for AVM intervention during pregnancy and puerperium as well as the best mode for AVM management were decided based upon maternal counselling and multidisciplinary opinion. The myths of embolization teratogenicity and vaginal delivery risk during pregnancy appeared true due to lack of congenital anomalies over 2 years follow up. Although our cases underwent caesarean section, there is no apparent risk in normal vaginal delivery.
Conclusion: Cranial AVM during pregnancy is an uncommon, yet a challenging topic. Small sample size and relatively limited period of follow–up were our major obstacles. Further prospective studies are needed to elucidate the safety of endovascular versus microsurgical management during pregnancy over a longer follow up periods, especially in context of embolization with non–adhesive embolic agents, such as Onyx.
Keywords: arteriovenous malformations, cranial arteriovenous malformations, arteriovenous malformations in pregnancy, avm rupture, aneurysmal haemorrhage, avm haemorrhage
Brain Arteriovenous malformations (AVM) are abnormal communication between parts of cranial arterial and venous systems with the lack of a true nutritive and absorptive capillary bed.1 The universal prevalence of AVM is relatively uncommon, with a prevalence ranging from 0.01–0.5%, 10/100000 to 140–500/100000 specifically2–7 clustering between 3rd and 5th decade i.e. presenting usually before 40 years old of age.4,8,9 AVM commonly presents with hemorrhage in more than one half of the cases even during pregnancy.4,8,10–17 Microsurgery, endovascular embolization and stereotactic radiosurgery are the well–established lines of management, applied on integrated and –sometimes– on individualized bases.1 With the exception of ARUBA trial, evidence is limited to case series and case studies for natural history and management.18,19 Moreover, 2 problems arise in the context of AVM management during pregnancy: first is the significant morbidity and mortality of intracerebral hemorrhage due to AVM or aneurysm during pregnancy and puerperium20 with estimated 4.4%21 or 5–12%.22 of all maternal deaths rendering it–one time– the third non–obstetric cause of maternal deaths21 second is the poorly defined natural history of AVM during pregnancy3,23 which hinders development of definite guidelines for management of AVM during pregnancy.3,4 In this case–series study along with a literature review, we try to establish a practical algorithmic management in addition to answering the frequently asked questions in obstetric and endovascular management.
Among AVM cases presenting to the emergency department of the Alexandria university hospital, cases presented with ruptured cranial AVM during pregnancy were selected. The diagnosis was established based on urgent MRI brain (contrast–enhanced) revealing intracranial hemorrhage, with MRA brain. Cases presenting with life–threatening hemorrhage underwent surgical evacuation of the hematoma.
In all cases, diagnostic subtraction angiography was done through femoral approach using 5 F diagnostic catheter with superselective angiography using Marathon (Covidien, Irvine, California, USA) and Mirage (Covidien, Irvine, California, USA) to reveal compartmental anatomy, flow related aneurysms, better feeder and draining venous anatomy and –finally– to rule out associated vascular anomalies.
Due to lack of clear guidelines regarding timing and choice of appropriate intervention (microsurgical resection versus endovascular embolization), counseling regarding these issues was the adopted policy, explaining– in details– the advantages and possible complications. In case of approval, endovascular embolization or microsurgical resection was performed.
For endovascular embolization, 6 F Guider Envoy catheter (Codman Neurovascular, Raynham, Massachusetts, USA) over a 0.38 inch wire was used. Superselective angiography is done using Marathon 1.5 (Covidien, Irvine, California, USA) microcatheter over a Mirage microwire 0.08 (Covidien, Irvine, California, USA). Embolization was performed using Onyx–DMSO (Covidien, Irvine, California, USA) and /or n–Butyl Cyanoacrylate Glue (NBCA) (B Braun, Melsungen, Germany). Post–procedural imaging and follow up modified Rankin Score as well as fetal/pediatric assessment for congenital anomalies (up to 2 years) were documented. For cases refusing intervention, the same follow up was performed besides the natural history of AVM regarding rebleeding or further symptoms. Neurosurgical, anaesthesia as well as obstetric consultation and feedback were done, aiming at multidisciplinary approach for the best delivery mode.
In addition, we conducted a review of literature (Pubmed, Sciencedirect and other databases) during the years 1972 to 2013 in order to answer common questions concerning the following:
We used the keywords: arteriovenous malformations, cranial arteriovenous malformations, arteriovenous malformations in pregnancy, AVM rupture, aneurysmal haemorrhage and AVM haemorrhage.
Among AVM cases presenting to the emergency department of Alexandria university hospital, 5 cases presented with ruptured cranial AVM during pregnancy with mean age of 29.2 years and different pregnancy trimesters at time of rupture (40% at 1st trimester, 40% 2nd trimester and one case in 3rd trimester). The clinical presentations included headache (100%), ataxia (40%), disturbed sensorium (40%), bulbar dysfunction (20%), hemiparesis (20%) and focal seizures with secondary generalization (20%).
Urgent MRI brain revealed intracranial hemorrhage in all cases (supratentorial lobar hemorrhage in 2 cases, cerebellar hemisphere in 2 cases and pure intraventricular hemorrhage in one case). DSA revealed that two cases were supplied exclusively by the carotid system (one via anterior cerebral artery and the other via anterior choroidal artery), 2 cases supplied by the vertebrobasilar system (on case supplied by hemispheric branch of posterior inferior cerebellar artery and the other is supplied by PICA, AICA and SCA) and only one case supplied in a mixed pattern (temporal branches of middle cerebral and posterior cerebral arteries). One case showed fistulous elements, one case showed flow–related aneurysms, no case showed perinidal angiogenesis (Figure 1 & 2).

Figure 1 Case 1: A 28 years old female patient presented during the second month of pregnancy with acute hemiataxia. MRI brain showed Left cerebellar hematoma. DSA showed Lt hemispheric cerebellar AVM supplied by hemispheric branches of Posterior Inferior Cerebellar Artery (PICA). Onyx and NBCA embolization was done in two sessions with eventual complete obliteration and marked symptomatic improvement 6 months later.
Conventional angiogram during embolization procedure: A: MRI shows resolving cerebellar hematoma, B&C: AP and lateral views of the left vertebral artery showing the Lt hemispheric cerebellar AVM nidus supplied by hemispheric branches of Posterior Inferior Cerebellar Artery (PICA) and superior cerebellar artery D: microcatheter is inside the AVM nidus. E&F: AP and lateral views showing disappearance of the nidus.
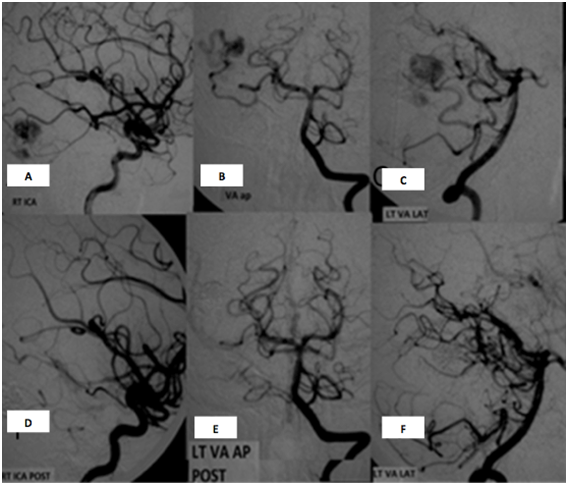
Figure 2 Case 2: A 24 years old female patient presented during the fourth month of pregnancy with ICH and IVH. The ICH was treated conservatively and patient recovered completely. Investigation revealed the presence of right temporal AVM supplied by Right MCA (middle cerebral artery) and Right PCA (posterior cerebral arteries). The AVM was Spetzler-Martin grade 2 with superficial venous drainage and size less than 3 cm. Onyx embolization was done. With complete obliteration using a single microcatheter in one session. The Patient recovered completely after embolization with the clinical and radiological follow up showing no recurrence and the patient was completely normal.
Conventional angiogram during embolization procedure: A: lateral view of the RT ICA showing the AVM nidus supplied by the temporal branch of the MCA. B&C: AP and lateral views of the VA showing an AVM nidus supplied by the temporal branch of RT PCA. D&E&F: corresponding views of the RT ICA, AP and lateral view of the vertebral artery angiogram showing disappearance of the nidus.
For the purpose of proper management, key concerns regarding management were reviewed along the literature, together with multidisciplinary consultation on individual case–by–case basis. These were: The effect of pregnancy as a risk for AVM rupture, the best time for AVM intervention during pregnancy and puerperium, the best mode for AVM management, as well as the myths of embolization teratogenicity and vaginal delivery risk during pregnancy to determine the safest mode of management in general and delivery in particular. After informed consent, 3 cases agreed to undergo endovascular embolization (using Onyx and or n–BCA) while 2 cases chose expectant management and follow up. Delivery by caesarean section was the obstetric management in all cases.
For risk of rebleeding, no rebleeding occurred in our cases over a 2 years follow up. Overall, AVM–induced hemorrhage represents 2% of all hemorrhagic strokes in the general population.10 Among known risk factors for hemorrhage in AVM (previous hemorrhage history, deep location, deep venous drainage and high feeding artery pressure)17,24–46 pregnancy still remains a debatable risk factor for first rupture of AVM during pregnancy.1,25 Although Swain and others report increased risk of 1st hemorrhage in unruptured AVM during pregnancy (87% in 24 pregnancies with AVM by Robinson et al, 9.3% by Forster et al.47) Horton et al.48 estimate prevalence of first AVM hemorrhage as 0.035 +/– 0.005/person–year in 451 pregnant females with unruptured AVM, which didn’t significantly differ from that of nonpregnant females during childbearing period (0.035 +/– 0.005/person–year). Also, Fujita et al.49 conclude a 0.065 +/– 0.036/person–year with similar non–significance.50 Consistent with these results, Walter, Sekhar et al.22 report a 3.5% rupture risk (similar to 2–4% risk of general population) as well as Finnerty & Martindale.51 It seems that pregnancy has a certain connection with cerebrovascular disease in general.1,52
Methodologic issues stand for explanation of this controversy, notably due to small sample size.12,49 along with the assumption of AVM being since birth for calculation of hemorrhage risk.25 On the other hand, pregnancy –as well as history of previous AVM rupture– is a known association with increased rebleeding from AVM, whether in the same pregnancy or later.10,12,49,51,53–55 Recurrence usually occurs in the 1st year from previous hemorrhage53 or even can recur in the same pregnancy.54 A number of mechanisms can explain such phenomenona, well illustrated in a review by Nitin et al.1 which are categorized under structural (high velocity feeder, lack of smooth muscles at nidus leading to loss of autoregulation and low nidal resistance, together with occasionally incomplete venous elastic lamina), hemodynamic (turbulence, shear stress, high pressure feeder effect) cardiologic and endocrinologic (vasoactive estrogen and progesterone) hypotheses, though no definite associations yet exist.
Of course, we have been anticipating results from ARUBA trial18,56 till it was recently halted by NINDS organization due to preliminary results showing superiority of conservative AVM management to interventional therapy, as the event rate was found to be 3 times higher in the intervention group than conservative arm.57 Improper external validity as well as internal one compromises the trial8 however ARUBA represents the idea of being the first possibly randomized controlled prospective trial for natural history and management of unruptured AVM including these occurring during pregnancy.
Temporal pattern of AVM rupture still remains a vague issue. No specific trimester of rupture was observed in our cases (Table 1). In retrospective series of Trivedi et al.22 the majority of AVM rupture during pregnancy occured in antepartum period (92%) rather than labour or puerperium3,22,24,58,59 some studies state a more specific timing, whether from the 20th gestational week to 6th week postpartum,21 the 2nd trimester47 3rd trimester22 both trimesters25 or –even– no specific predilection to any trimester at all10 The retrospective study design can explain the conflict among these extrapolated timings.
|
Case |
Age |
Gestational month and trimester |
Presentation |
imaging |
Feeder |
Type/ draining pattern |
Intervention done |
Modified rank in score at 90 days |
|
1 |
24 y |
4th month |
-Headache |
Rt temporal hematoma 4th ventricle IVH |
Temporal branches of Rt PCA and Rt MCA |
-Sulco-gyral |
Endovascular embolization using 1 amp of Onyx During 2nd month |
1 |
|
2 |
28 y |
2nd month |
Acute hemiataxia |
Lt cerebellar hematoma |
Hemispheric branch of Lt PICA |
-Gyral |
Endovascular embolization using 1 amp of Onyx |
2 |
|
3 |
35 y |
8th month |
Hemiataxia Bulbar palsy |
Rt cerebellar hematoma and IVH 4th ventricle |
Rt SCA, Rt PCA, Rt PICA and Rt AICA |
-Gyral |
Endovascular embolization using 5 amp of Onyx. During 8th month |
3-4 |
|
4 |
32 |
2nd month |
Headache |
ACA internal frontal branch |
-Sulco-gyral |
Conservative Uneventful during |
0 |
|
|
5 |
27 |
4th month |
Headache |
Pure IVH |
A choroidal |
-Plexal |
Uneventful during pregnancy and 2y follow up |
0 |
Table 1 The patients' characteristics
Consistent with literature, Age at the time of rupture during pregnancy averaged around the 3rd decade (Table 1). AVM generally is discovered between the 3rd and 5th decades, with hemorrhagic presentation in more than half of cases. Among pregnants presenting with intracranial hemorrhage, Velut et al observed that hemorrhage from AVM occurs at younger age than aneurysmal hemorrhage.60
Based upon the previous literature and our series, we can conclude that pregnancy isn’t a definite risk factor for AVM rupture, yet it’s a possible risk factor for AVM rebleeding. The majority of intracranial haemorrhages occurs during the antepartum period (Figure 3).
Due to vague natural history, patient counselling usually dominates the clinical decision. Consistent with the controversial risk of AVM rupture during pregnancy, the safest management overall is preconceptual intervention.12,25,60–63 The true problem is the discrimination of elective versus emergency management during pregnancy, a problem encountered in 2 of our cases, with cerebellar hematoma. Considering Spetzler–Martin scale64 the cases represent 3 ruptured AVMs with a small size (which explains the high risk of rupture65 yet it carries the advantage of minimizing the number of interventional sessions) and situated in the posterior fossa (which carries a higher morbidity by its eloquence).66,67 So rupture status, posterior location– together with the size– were the determinants among which we decided urgent timing of intervention. Emergency management is reserved for ruptured AVM, especially if clinically unstable.23,61 One study recommends emergency intervention only for unstable ruptured cases.61 nevertheless, the safer advances in microsurgery and endovascular techniques relative to the publication date of the previous paper renders this decision a relatively obsolete one.60,62
The most problematic decision– however– is the management of unruptured pregnant cases. While maternal and foetal risks of radiation or anaesthesia are now minimized in comparison to risk of rupture63 yet the opponents of early management use the less definite risk of first hemorrhage to recommend treating unruptured AVM on postpatum basis, similar to aneurysmal management during pregnancy.60,61 To date, the level V case series and retrospective studies fail to show preponderance of a certain management line, making counselling the only available tool. Ogilvy et al.11 state that counselling won’t favour elective treatment after delivery with risk–benefit analysis.12 Till more prospective studies with adequate sample power develop, risk–benefit counselling remains the gold standard management of AVM intervention timing whether preconceptually or during pregnancy. The only exception is the emergency management of ruptured AVM, especially in clinically unstable patients (Figure 4).
For the choice of the best modality of intervention, we considered surgical management in only one case with cerebellar hematoma evacuation (Table 1) Surgery used to be the gold standard management of AVM overall, even during pregnancy, due to its reported association with lower foetal and maternal mortality.2 However, the anaesthesia, patient positioning and approach considerations (vide infra) made the sole reliance on surgical management a real challenge. Advances in microsurgery as well as adjuvant endovascular AVM intervention are rather options of choice nowadays62 especially Spetzler–Martin IV and V lesions, which need a multidisciplinary management.1
Similar to the evolution of surgical concept, the endovascular management of AVM used to be contraindicated in pregnancy due to foetal and maternal radiation risks. In contrast to stereotactic radiosurgery, the radiation used in endovascular embolization falls mostly short of teratogenicity.63 First, Marshman et al.68 report a 30–45 minute radiation exposure of 0.17 to 2.8 mGy69 well below the teratogenic threshold (estimated to be 100 mGy at 0–8 embryonic days, 120 mGy at 8–15 weeks, 250 mGy at 16–25 weeks and 500 mGy at 2–8 weeks & 25th week onwards). In addition, Onyx and DMSO materials –being inert molecules– have not yet proved to be teratogenic.70,71 Besides, the swiftness of AVM obliteration with endovascular management renders it a rather attractive emergency and safer option than radiosurgery. Consistent with these results, our cases reported no congenital anomalies at all over 2 years of follow up.
In comparison to surgical management, Stereotactic radiosurgery remains the least favorable modality for AVM management during pregnancy. Not only due to radiation risks1,63 but also due to prolonged latency for effective therapeutic obliteration and hence reduction of hemorrhage risk.72,73 Late trials state the 2 year complete obliteration rate as 80–84%73,74 compared to 14–90% using combined SRS and Endovascular approaches.75 90% of AVM obliterations occur between 24 and 58 months period68 for these reasons (mainly for safety), we didn't recommend stereotactic management at all in our cases.
So, the decision of embolization versus microsurgical management should be based on integration and individualization rather than solely comparing the two modalities, missing the better of two worlds. Endovascular embolization can be a perioperative antepartum modality for future microsurgical resection in anaesthetically safer conditions, or –in individually selected cases– a primary modality of intervention during pregnancy (Figure 3). We think there’s no consensus for selection criteria, rendering the selection on operator–experience basis in addition to maternal counselling. Also, The best mode for AVM management during pregnancy should be individualized (Figure 5).
In view of a poorly defined natural history23 no definite guidelines exist for the best delivery mode.76 Some report caesarean section as a preferred mode to avoid stress and straining as risk factors for AVM hemorrhage22,23,77–79 and there is a case report about simultaneous caesarean section and AVM microsurgical resection.3 However, several issues stand against such a choice: first, all these recommendations are based on expert–opinion case reports. Second, Valsalva maneuver is proved not to directly transmit pressure to the draining veins.80 Most importantly, Labour and puerperium are not a great risk periods for AVM rupture (vide supra), thus rendering caesarean section no safer than vaginal delivery.22,30 Counselling still remains mandatory in these situations.1 Although our cases underwent caesarean section (either due to anaesthetic considerations or due to patients' preference), we think that decision of delivery should be based on obstetric rather than anaesthetic or neurosurgical basis (Figure 6).
Brain AVM during pregnancy is an uncommon, yet a challenging topic. Small sample size and relatively limited period of follow–up were our major obstacles. Further prospective studies are needed to elucidate the safety of endovascular versus microsurgical management during pregnancy over a longer follow up periods, especially in context of embolization with non–adhesive embolic agents, such as Onyx.81–86
None.
The authors disclose no conflicts of interest.
This article was presented in XXX conference in Japan and an abstract has been submitted to European Society of Minimally Invasive Neurologic Therapy conference in Nancy, France.

©2016 , et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.