Journal of
eISSN: 2373-6410


Case Report Volume 4 Issue 6
1Department of Neurology, Instituto Nacional de Neurología y Neurocirugía, Mexico
2Department of Neuroinfectology, Instituto Nacional de Neurología y Neurocirugía, Mexico
Correspondence: Graciela Cárdenas, Instituto Nacional de Neurología y Neurocirugía Manuel Velasco Suárez Department of Neuroinfectology. Insurgentes Sur 3877, Col. La Fama, 14269, Mexico
Received: December 03, 2015 | Published: June 16, 2016
Citation: Espinoza-López DA, Soto-Hernández JL, Cárdenas G (2016) Cortical blindness (Anton-Babinski Syndrome), an Unusual Manifestation ofCentral Nervous System Tuberculosis. J Neurol Stroke 4(6): 00157. DOI: 10.15406/jnsk.2016.04.00157
Anton syndrome, or cortical blindness, consists of an objective blindness in the presence of an intact anterior visual pathway, with the patient expressing a denial of blindness; it is a peculiar form of visual anosognosia. Those patients with this syndrome often show an inappropriate affect and confabulate to conceal their visual loss. The common pathologic process in Anton syndrome is an ischemic event of the occipital circulation, and the most frequent causes are cerebral infarctions in aged patients with vascular risk factors, while hypertensive encephalopathy, eclampsia, hypoperfusion, trauma, tumors, and bacterial meningitis are common in children. Herein we report two patients with Anton-Babinski syndrome related to tuberculous meningitis, cared for at a single center. A search in the literature did not render any similar cases previously reported. The patients had no previous history of vascular risk factors, and the presentation of tuberculous meningitis was atypical: in the context of a rapidly evolving headache, one of the patients had visual changes and mental confusion; the other patient presented with stupor caused by acute hydrocephalus. Ischemic events were documented by imaging studies, and cerebrospinal fluid was inflammatory with positive polymerase chain reaction (PCR) for Mycobacterium tuberculosis and high adenosine deaminase levels. Antituberculous drugs were administered and both patients stabilized; one patient showed no significant visual recovery, while the other was able to walk unassisted but had severe and incapacitating neuropsychiatric sequels at 3 years of follow-up.
Keywords: Cortical blindness, Central nervous system tuberculosis, Vasculitis
Tuberculosis (TB) is the world’s second most common cause of death by infectious disease after HIV/AIDS. It remains as a major health threat with high morbidity and mortality. According to World Health Organization estimates, one-third of the world’s human population is latently infected with Mycobacterium tuberculosis (Mtb), and 1.3 million people die of TB each year.1 One of the most feared complications of TB is the involvement of the central nervous system (CNS) (meningitis, tuberculomas, and abscesses). The outcome of CNS TB is usually poor in most cases, in direct correlation with the stage severity according to the British Research Medical Council.2,3
Anton syndrome, or cortical blindness, was first described by Gabriel Anton in 1886, who presented patients with objective blindness and deafness, which showed a lack of self perception on their deficits. Later, Joseph F. Babinski used the term anosognosia to describe this phenomenon.4,5
Bilateral damage of the occipital lobe can be induced by several conditions, such as hypoxia, vasospasm, or cardiac embolism,6 but cerebrovascular diseases are the main causes.5 In turn, cerebrovascular diseases can occur as a complication of a variety of CNS infections leading to vasculitis, primarily affecting the vessels at the base of the brain in the setting of meningitis.7
CNS vasculitis refers to the inflammation and destruction of the brain-, spinal cord-, and meningeal-blood vessels. It can be classified into primary and secondary vasculitis. The former occurs when injury is confined to the CNS and no systemic involvement is observed, while the latter results in CNS affection in the context of a systemic inflammatory or infectious process.8 The spectrum of diseases demonstrably associated with prominent vascular inflammation in the CNS includes bacterial, fungal, viral, and protozoal infections. Inflammation of the vasculature may develop either from extension of neighboring foci or reflect a direct invasion of pathogenic microorganisms in the vessel walls.9
Herein we present two cases of tuberculous meningitis (TBM) with an atypical neurologic manifestation (bilateral occipital lobe infarcts, Anton-Babinski syndrome) related to infectious vasculitis.
A 44-year-old man, who worked as a car repairman, was presented to the hospital with a 4-day illness characterized by progressive headache, followed by bilateral blurred vision, confusion and somnolence. Vital signs were BP 140/80, HR 78X’, RR 18X’, and temperature 36.8 °C. Laboratory studies reported hemoglobin 17.9, hematocrit 52.1%, WBC 14,310/mm3, 71% neutrophils, platelets 277,000/mm3, and glucose 115mg/dL, BUN 14mg/dL, total cholesterol 194mg/dL, and triglycerides 147mg/dL. On neurological examination upon admission the patient was alert, prone to sleepiness and inattentive, with fluent language; he was able to repeat, nominate, and follow simple commands. He was disoriented in time, with denial of his illness. On ocular examination no perception of light in both eyes was found, but corneal reflex was present. Pupils were symmetrical and reactive to light and accommodation, ocular movements were preserved, but optocinetic nystagmus could not be elicited. The other cranial nerves were normal, dysdiadochokinesia was present and no meningeal signs were found. Psychiatric evaluation reported that he was disoriented in time and circumstances, with consistent speech, without hallucinatory behavior but unable to recognize blindness. On the following days he presented labile affect and greater confusion. On CT scan of the head and magnetic resonance imaging, subacute infarcts in the territories of posterior cerebral, anteroinferior cerebellar, superior cerebellar and thalamic arteries were found (Figure 1A), consistent with a diagnosis of cortical blindness. After gadolinium administration, an enhancement of basal cisterns and cortical occipital lobes was demonstrated (not shown); the latter finding prompted at 11 days post admission to a lumbar puncture, which yielded xanthochromic fluid containing mononuclear cells per mm3, protein level of 367mg/dL and glucose of 65mg/dL, with serum glucose of 108mg/dL. CSF adenosine deaminase determination was 9 U/L; stains and cultures for bacteria, mycobacteria, and fungi were negative. Polymerase chain reaction (PCR) for Mycobacterium tuberculosis in CSF was positive, and serum VDRL and HIVELISA tests were negative. All other evaluations, aiming to discard alternative causes of stroke, including transesophagic echocardiogram, cerebral angiography and protein C and S determinations, as well as antibodies to hepatitis B and C and antinuclear antibodies, were normal or negative. Treatment with 4 anti tuberculous drugs was started and the patient was discharged 15 days after admission. In the outpatient clinic, 3 weeks later, he was able to perceive some simple figures. Two months later het was reassessed by ophthalmology, with no major changes.
A 40- year-old construction worker presented with a 2-day history of fever and rapidly progressive headache, accompanied with photophobia, unsteady gait, and vomiting; on the next day he developed incoherent speech and a rapid deterioration of consciousness. He arrived stuporous to another health-care facility, where he underwent intubation and mechanic ventilation; a cranial tomography showed severe hydrocephalus. He was transferred to our institute. Upon admission, neurological examination revealed decerebration and rigidity with painful stimulus. Vital signs were: BP 111/67, HR 64X’, RR 14 X’, and temperature 35.8 °C. Laboratory studies reported hemoglobin 17.1, hematocrit 49.8%, WBC 13, 950/mm3, 82% neutrophils, platelets 226,000/mm3, and glucose 215mg/dL; HIV test was negative. The patient underwent immediate surgery, with placement of a right precoronal ventriculostomy. Ventricular cerebrospinal fluid revealed glucose 94mg/dL, protein level 12mg/dL and cell count 6 cells/mm3. CSF cultures for bacteria and fungi, India ink test, and latex for Cryptococcus were negative. Three days later, the ventricular drainage system was changed for an internal CSF derivation. The patient remained somnolent and began to present alternative periods of eye opening with inattention and a fixed staring. Pupils were symmetrical, 3-mm, nonreactive to light. An ophthalmological evaluation 6 days after admission, when the patient issued a few words, identified no light perception on the left eye and finger counting at 30cm on the right eye. Papillae were well defined, and the foveolar reflex was present bilaterally. Metilprednisolone was indicated and 1-gram boluses were administered, without visual changes. CSF obtained by a lumbar puncture after hydrocephalus was controlled was xantocromic. Cell count was 304 cells per mm3 (60% mononuclear leukocytes and 40% polymorphonuclear leukocytes) with protein level of 212mg/dL, and glucose level of 65mg/dL, with simultaneous blood glucose level of 222mg/dL. Microbiological stains for bacteria, fungi, and mycobacteria, as well as cultures, were negative. The CSF adenosine deaminase level was 22U/L. With these results, intravenous dexamethasone and a 4-drug antituberculous treatment was started; a few days later, a CSF-PCR test for Mycobacterium tuberculosis was positive. MRI showed hyperintensity on occipital regions, extending toward the parietal lobe on the left side, and more localized to the calcarine region on the right side; diffusion restriction was present (Figure 2a & 2b), and peripheral enhancement after gadolinium administration was observed (Figure 2c). The patient was discharged five weeks after admission, and slowly recovered language and referred complex visual hallucinations and mood lability, requiring support by relatives and neuroleptics. He completed one year of antituberculous drug treatment. Two years later, he was able to ambulate without assistance, and a campimetric evaluation reported homonyms, vision islets. A control CT scan (Figure 2d) obtained 29 months after hospitalization showed bilateral occipital infarctions with functional CSF shunt.
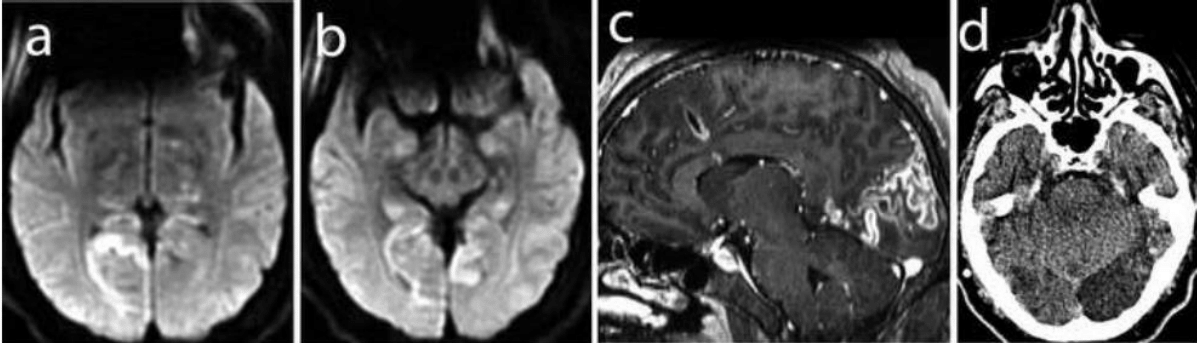
Figure 2 Patient 2: 2a and 2b Axial MR at admission (FLAIR sequences) show bilateral hyperintensities at the occipital cortex. 2c Sagittal MR with gadolinium at admission shows early luxury perfusion at the occipital cortex 2d Axial CT scan at 29 months after admission shows partial recovery of the ischemic occipital cortex.
Tuberculosis remains as a major health threat, with high morbidity and mortality. The main trait of CNS-TB is the wide range of clinical manifestations and severe sequels, which constitute a huge challenge for physicians, requiring accurate diagnosis and early treatment. Cerebral vessel damage and brain infarcts in tuberculous meningitis have long been recognized.10,11 Severe vascular complications occur in 15-57% of patients, especially in advanced stages of infection and severe illness. Most strokes are asymptomatic since they occur on a silent area, but deep coma may be a single manifestation of brainstem infarctions. Most strokes in TBM are multiple, bilateral, and are located in the basal ganglia, especially in the “tubercular zone” that comprises the caudate, anterior thalamus, anterior limb, and genu of the internal capsule. These territories are supplied by the medial striate, thalamotuberal and thalamostriate arteries.11 It is assumed that this topographical distribution is closely associated with the inflammatory meningeal exudate, although it has also been considered that these vessels could be stretched by hydrocephalus.12
Caseating granulomatous inflammatory exudates in tuberculous meningitis affects predominantly the basal cistern. This exudate can block the CSF flow, resulting in frequent cranial nerve palsies and obstruction of the vessel traversing CSF, leading to obliterate vasculitis, cerebral infarction, and hydrocephalus.13 Another mechanism, possibly involved in the development of infarcts is a secondary necrotizing panarteritis. This process initially involves the adventitia and then gradually spreads, conditioning secondary thrombosis and occlusion.15
Three main pathological mechanisms leading to vascular damage have been proposed: infiltrative, proliferative, and necrotizing. Infiltrative involvement (vasculitis) occurs from the adventitia inwards, and it is most prominent in vessels passing through the basilar exudate. Proliferative changes consist of intimal layer thickening, with resultant stenosis or occlusion.
Necrotizing vascular lesions in tuberculous meningitis may affect any of the vessel layers, and it may occur either with or without infiltrative involvement. The exact pathologic mechanisms involved in tuberculous meningitis-associated stroke remain unresolved.7
In some instances, infarction occurs in the absence of parent vessel changes (or thrombosis), raising again the possibility of vasospasm as a potential mechanism, especially in the early course.
Ischemic complications are an important predictor of the clinical outcome in TB.16 Patient mortality shows a three-fold increase with respect to those individuals without infarcts, as well as the extension of ischemic cerebral lesions.16,17 A study using transcranial color 9 coded sonography (TCCS) and magnetic resonance angiography (MRA) reported 15 patients with tuberculous and cryptococcal meningitis; 4/15 (27%) exhibited stenosis of at least one cerebral artery, while three had bilateral middle cerebral artery (MCA) stenosis, and one had unilateral MCA stenosis. After a 6-month follow-up, fatality rate was 50% (2/4) in patients with intracranial arterial stenosis and 9% (1/11) in patients without this condition. The risk of poor outcome at a 6-month endpoint had an odds ratio of 5.3 for patients with intracranial arterial stenoses with respect to those without the sign. The authors comment that in chronic meningitis, initially the cerebral blood flow (CBF) increases significantly due to metabolic disturbances, including decreased intra-cerebral interstitial pH, increased CSF lactate formation, and released vasoactive substances involving inflammatory host reactions. As the meningitis progresses, the inflammatory changes result in endothelial dysfunction and the consequences include loss of cerebrovascular auto-regulation and increased permeability of the blood-brain barrier. Finally, a decrease in CBF becomes apparent in relation to increasing intracranial pressure, developing vasculitis that leads to arterial stenosis and cerebral ischemia.18
Several infectious (viral, bacterial, fungal, and parasitic)19-26 and non-infectious causes are related with ischemic cortical blindness,27,28 but despite of the high frequency of vascular complications in TBM11,17,29 only one case of cortical blindness (related to PRES, a primarily vasogenic edema of the subcortical matter with a predilection for the parenchyma supplied by the posterior circulation) in a HIV-patient has been reported.30 In that case, the patient had a low CD4 count, 29 cells/μL, and high HIV-RNA viral load > 750,000 copies, with disseminated tuberculosis; another important characteristic was the absence of CSFinflammation in the lumbar puncture.30 In our report we presented two patients with TBM; vasculitis was demonstrated in one of them. Our cases add information to the very limited literature on the Anton syndrome, especially in relation with cerebrovascular complications in TBM. In this context, the suspicion of cortical blindness should be raised in patients with atypical visual loss and evidence of ischemic occipital lobe lesion. Regarding CNS-TB, it is important to make an early recognition and to start empiric therapy in other to prevent severe complications such as vasculitis, infarctions, and death. Tuberculosis should be considered in the differential diagnosis of stroke or hemorrhagic complications in patients living in endemic areas, even in the absence of inflammatory CSF characteristics, as well as disseminated involvement.
None.
None.

©2016 Espinoza-López, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.