Journal of
eISSN: 2373-6410


Research Article Volume 13 Issue 4
Clinical Neurologist Master’s Student in Medical Sciences Research at UNAM, Mexico
Correspondence: Cecilia Sandoval Carrillo, Clinical Neurologist Master’s student in medical sciences research at UNAM, Mexico
Received: July 15, 2023 | Published: August 1, 2023
Citation: Carrillo CS. Cognitive impairment secondary to cerebral circulation disturbances detected by transcranial doppler in patients with lupus systemic erythematosus. J Neurol Stroke. 2023;13(4):82-95. DOI: 10.15406/jnsk.2023.13.00550
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease of unknown etiology with heterogeneous clinical manifestations including neuropsychiatric manifestations, the prevalence of which is unknown (14-75%). However, it is known that patients with SLE develop cognitive impairment even if they do not present other neurological manifestations clinically. In most patients, intellectual impairment is subclinical and is revealed by neuropsychological testing. The pathogenic factors associated with and triggering cognitive impairment in SLE are still unknown and it is very likely that it is multifactorial; essentially the following lines of research are being pursued (Figure 1): that it is a consequence of the production of autoantibodies directed to components of brain tissue, that inflammatory mechanisms are involved by cytokines in the presence of damage to the blood-brain barrier, or that cerebrovascular alterations associated mainly with microangiopathic processes participate. Although most studies are focused on demonstrating the autoimmune mechanism, there is evidence that allows us to support the participation of the vascular mechanism and currently there is transcranial Doppler technology that allows us the noninvasive evaluation of cerebral circulation in an easy, fast and economically accessible way. Therefore, it is essential to increase the knowledge about the pathogenic mechanisms responsible for cognitive alterations in SLE, so it is widely justified to carry out a research project to evaluate the state of cerebral circulation in patients with SLE and determine its potential involvement in the development of cognitive impairment associated with systemic lupus erythematosus. To evaluate the cerebral circulation, transcranial Doppler technology was selected to study in a simple, non-invasive and reproducible way, at low cost and in real time different parameters of the hemodynamics of the cerebral circulation including measurement of blood flow velocities and cerebrovascular reactivity (CVR) test that determines the state of the cerebral microcirculation.
Objectives: To establish the prevalence of cognitive impairment in a cohort of SLE patients and to determine the frequency and magnitude of cerebral circulation alterations (blood flow velocities, pulsatility, and cerebrovascular reactivity) measured by TCD in patients with systemic lupus erythematosus and cognitive impairment, compared with SLE patients without intellectual impairment and with healthy control subjects.
Methods: Case-control study nested in a cohort where patients with SLE belong to a cohort of 139 patients from the Department of Immunology and Rheumatology of INCMNSZ, cohort that is part of the protocol "Predictors of cognitive dysfunction in patients with generalized lupus erythematosus". Neuropsychological tests were applied in these patients to establish the presence of cognitive impairment. There were three study groups: (1) the problem group were patients with SLE and cognitive impairment; (2) control group 1 were patients with SLE without cognitive impairment; and (3) control group 2 were healthy controls assumed to have normal intellectual function. In all three groups, evaluation of the cerebral circulation was performed using transcranial Doppler technology, with recordings from the middle cerebral artery to obtain various parameters of the hemodynamics of the cerebral circulation including measurement of blood flow velocities and CVR; in this test the ability to increase blood flow velocities to hypercapnia by producing vasodilatation of the cerebral microcirculation is estimated (hypercapnia is provoked by inhalation of a mixture of CO2 at 8%, O2 at 21% and nitrogen balance for one minute - administered through a mask).
Results: Of 67 patients with SLE included in the study, 29 were found to have alterations in the evaluation of cognitive functions for a prevalence of 43% in this cohort; cognitive impairment was categorized as mild in 16 patients (23.9%), moderate in 6 (9.0%) and severe in 7 patients (10.4%). When evaluating the hemodynamics of the cerebral circulation determined by transcranial Doppler in patients with SLE according to the presence of cognitive impairment and with healthy control subjects it was found that while the snguine flow velocities and pulsatility index showed no differences, significant alteration of cerebrovascular reactivity was found in patients with SLE and cognitive impairment (increase of only 13.1±9% to hypercapnia, compared to increase of 24.2±18.2% in patients without cognitive impairment; p=.01). When including in the analysis the group of healthy control subjects and stratifying the SLE patients according to cognitive performance in the neuropsychological tests, a gradient was found in the degree of alteration of cerebral vasomotor reactivity with greater deterioration of intellectual functions, such that the mean CVR±SD of increase to hypercapnea in the healthy control group was 32.6±16.5%, compared to 24.7±18.3%, 14.3±15.3% and 8.3±12.4 in patients with SLE and normal cognition, mild and moderate/severe impairment, respectively (p<0.001). When adjusting for cerebrovascular reactivity through multivariate analysis by binary logistic regression with other independent factors related to cognitive impairment, CVR was found to remain significant reaching the association estimate odds ratios of 9.8 (95% CI 2.9 - 32.8; p<0.001).
Conclusions: The prevalence of cognitive impairment in this SLE cohort was 43% and was associated with significant alteration of cerebrovascular reactivity, suggesting that vascular mechanisms that damage cerebral microcirculation are involved in the development of cognitive impairment in SLE patients.
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is a chronic inflammatory disease of autoimmune nature, of unknown etiology in which there is cellular and tissue damage due to autoantibodies and which presents with a wide spectrum of clinical manifestations. In 90% of cases it affects women of childbearing age, although it can also occur in childhood, in later decades and in men. The disease is multisystemic, although initially only one organ may be affected. Most patients follow a chronic course and have flares or exacerbations of the disease, interspersed with periods of inactivity.1
The incidence and prevalence of SLE vary according to the geographic area and ethnicity analyzed. Worldwide, the incidence is estimated to be approximately 1 to 10 cases and the prevalence 40 to 150 cases per 100,000 inhabitants, respectively.2–5 It affects certain ethnic groups more frequently and severely, being more prevalent in African Americans and Hispanics. In the United States of America, people of African, Hispanic or Asian descent, compared to other ethnic groups, tend to have a higher prevalence of this disease and greater vital organ involvement.6
The inflammatory process may involve skin, kidneys, lungs, serous membranes, joints, lymphatic system, as well as central and peripheral nervous system. In the last decades a remarkable improvement of the survival rate has been observed. The 4-year survival in 1950 was 50%, now it reaches 80% at 15 years.4,7 Due to the improvement in the detection of mild disease, from the clinical point of view, the incidence has tripled in the last 40 years.8
Neuropsychiatric systemic lupus erythematosus erythematosus
Survival of patients with SLE has improved markedly in recent decades, revealing that a proportion of patients develop neurological and psychiatric symptoms, known as neuropsychiatric SLE (NP-SLE). These neuropsychiatric manifestations can be classified as primary, which are those related to direct immunological or vascular damage to the nervous system, caused by the immunological disease (SLE); or secondary to systemic complications of the disease with repercussions on the nervous system, for example infectious processes, metabolic alterations, severe systemic arterial hypertension or associated with the use of drugs to control some aspect of the disease. A source of controversy is the attribution of NP manifestations to SLE, as it is sometimes difficult to establish whether the symptoms of SLE-NP are primary or secondary. Also, longitudinal studies have shown that NP manifestations can appear at any time in the course of SLE and there are no identified markers to predict which patients will develop them.3 Therefore, the understanding of SLE-NP is complex and is one of the main challenges in the study of SLE.
In order to unify criteria for the study of the various neurological and psychiatric manifestations that occur in patients with SLE, in 1999 the American College of Rheumatology (ACR) developed a nomenclature of Neuropsychiatric Lupus; it recognized 19 syndromes, 12 of the central nervous system (CNS) and 7 of the peripheral nervous system (Table 1). Despite the use of this common classification that unifies and defines specific clinical, laboratory and imaging or psychological testing criteria for each syndrome, the prevalence of neuropsychiatric syndromes is highly variable; according to a meta-analysis by Unterman et al.10 the prevalence ranged from 37% to 95% of patients with SLE. In a sub-analysis of this meta-analysis of 10 high quality prospective studies the estimated prevalence was 56.3% (95% CI 43 - 75%), 90% attributable to CNS involvement and only 10% to peripheral. The most frequent NP syndromes were headache (28%), depression (21%), cognitive dysfunction (20%), epileptic seizures (10%) and cerebrovascular disease (8%).10
|
Central nervous system |
Peripheral nervous system |
|
Aseptic meningitis |
Cranial neuropathy |
|
Cerebrovascular disease |
Mononeuropathy (single or multiple) |
|
Demyelinating syndrome |
Polyneuropathy |
|
Headache |
Inflammatory Polyradiculoneuropathy |
|
Movement disorder |
acute demyelinating |
|
Myelopathy |
Myasthenia gravis |
|
Epileptic seizures |
Autonomic neuropathy |
|
Cognitive dysfunction |
Plexopathy |
|
Acute confusional state |
|
|
Anxiety disorder |
|
|
Depression |
|
|
Psychosis |
Table 1 Neuropsychiatric syndromes in patients with SLE according to the classification of the American College of Rheumatology9
In patients with SLE, in the presence of neuropsychiatric manifestations, the first step in the diagnostic approach is to rule out a secondary cause (uremia, drugs, etc.) or associated cause (thrombotic thrombocytopenic purpura). Consequently, neurolupus represents a diagnostic challenge, since none of the syndromes described is exclusive to SLE and up to 40% are attributed to other causes. A number of laboratory and laboratory tests can aid in the diagnosis, but these should be used based on the patient's manifestations. In patients with manifestations of CNS involvement, magnetic resonance imaging (MRI) is the study of choice since it allows detecting focal lesions in the subcortical and/or periventricular white matter (15-60%), hyperintensities in the gray matter (24-30%), atrophy, ventricular dilatation and infarcts, although 30-40% of SLE-PNL have normal MRI.11,12 Despite advances, imaging studies do not allow differentiation between active and inactive disease, and the findings are not specific. Of greater relevance is that in case secondary causes are ruled out, the possibility of primary neurological involvement is high. Regarding prognosis, despite therapeutic advances, mortality in cases of neuropsychiatric SLE amounts to 7-19%. In particular, the presence of seizures, cerebrovascular disease and delirium are considered as markers of poor prognosis.13
Pathogenesis of neuropsychiatric SLE: The pathogenesis of SLE-NP is particularly complex and despite decades of research, understanding of the precise mechanisms remains limited. It is unlikely that a single pathogenic pathway can explain the diversity of neuropsychiatric manifestations. It has been postulated that genetic, neurochemical and environmental factors contribute to the development of immune dysfunction in SLE patients and that interrelated mechanisms must be involved including blood-brain barrier dysfunction, cerebrovascular involvement as well as autoantibody-mediated neuronal damage and proinflammatory cytokines (Figure 1).14,15
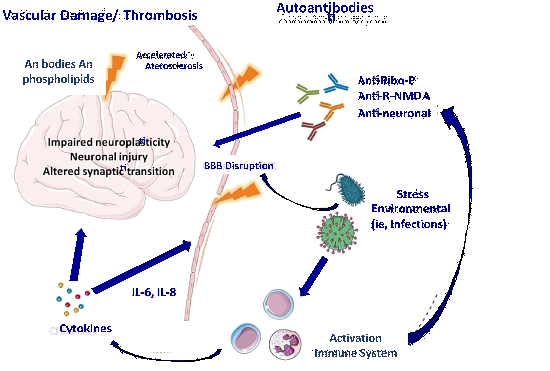
Figure 1 Proposed pathogenesis of SLE-NP. Auto-antibodies enter the brain causing neuronal damage, including impairment of neuroplasticity and synaptic transmission. To gain entry into the brain there must be at least transient disruption of the blood-brain barrier (BBB) by external (e.g., infections) or internal triggers (e.g., cytokine inflammation, metabolic disturbances). Vascular damage may be antibody-mediated through antiphospholipid antibodies and/or early development of atherosclerosis. Ribo-P = ribosomal P; R.NMDA = N- methyl-D-aspartate recpetor.
Even without obvious CNS symptoms, the brain of SLE patients shows atrophy and cortical and subcortical functional alterations of undetermined origin. Several autoantibodies have been associated with SLE-NP including anti-P ribosomal P antibodies, antibodies against neurofilaments, against microtubule-associated protein 2, antibodies against the N-methyl-D-aspartate (NMDA) receptor, endothelial cells and neuronal cells.14–17 Evidence from other autoimmune diseases indicates that certain autoantibodies can directly cause neuronal damage by interfering with the function of cell surface elements such as receptors and channels, Ions.18 In SLE, this possibility has been suggested by the finding of a subtype of double-stranded DNA autoantibodies that cross-react with the NMDA receptor and induce neurotoxicity and cognitive alterations.19
Anti-P ribosomal autoantibodies have long been considered among the candidates for a neuropathogenic explanation in SLE.20–23 In 1987, Bonfa et al. made the observation that anti-P antibodies are associated with psychosis in lupus.20 Some studies have confirmed the clinical association between anti-P antibodies and SLE-PN including their detection in the cerebrospinal fluid (CSF) of these patients.23 Anti-P antibodies recognize a novel integral membrane protein of the neuronal surface. In the brain, this neuronal surface protein P antigen (NSPA) is preferentially distributed in areas involved in memory, cognition and emotion. NSPA protein has been found to be expressed in neocortical layers II, V, VI and in other areas of relevance to the pathogenesis of SLE- NP, including the amygdala which is involved in consciousness and emotion,24 in the cortex and hippocampus, which are involved in memory and complex brain functions.25
Other autoantibodies potentially related to SLE-NP are anti-NR2. De Giorgio et al. found that an injection of anti-NR2 glutamate receptor binding antibodies (antibodies purified from serum of SLE patients, or from CSF samples of patients with SLE and progressive cognitive impairment) into the mouse brain resulted in apoptosis of neurons without signs of inflammation.26 The effect of anti NR2 antibodies is dose-dependent; at low concentrations, they alter synaptic function, whereas at high concentrations they cause neuronal death by apoptosis.27 It is recently highlighted that mice induced by antigens to express anti-NR2 have no neuronal damage until the blood-brain barrier is damaged.28 An intact blood-brain barrier prevents the transport of anti-NR2 from the systemic circulation to the brain.
In conclusion, one of the most current models explaining cognitive impairment in SLE-NP requires the presence of anti-DNA and anti-NR2 antibodies, which are present in 25-50% of SLE patients, as well as disruption of the blood-brain barrier, which may occur as a consequence of disease activity, for example in cerebral vasculitis, but may also be secondary to infections, stress, catecholaminergic excess, nicotine exposure, etc.29
On the other hand, vascular mechanisms that directly explain focal neuropsychiatric manifestations such as cerebral infarcts and seizures should be taken into account, and could have an important contribution in diffuse manifestations such as cognitive impairment. Autopsy studies indicate that vasculopathy is consistently present in CNS damage in patients with PN-SLE.29–33 Non-inflammatory small vessel vasculopathy, microinfarcts and multifocal microhemorrhages are common findings. Microangiopathy was initially attributed to immune complex deposition but the involvement of complement activation is now clearer. In the brain, the cause of cerebral infarcts and microhemorrhages appears to be caused by altered cerebral blood flow. Likewise, it is very likely that antiphospholipid antibodies are directly involved in the development of cerebrovascular damage. AAF are a consistent finding in patients with SLE-PN.(34) It is well known that these antibodies due to their affinity to phospholipids favor thrombotic events and these elements in combination constitute the antiphospholipid syndrome (APS). These patients have an increased risk of CVD either in the form of cerebral infarction, intracranial hemorrhage or venous sinus thrombosis. It is also striking that different series report the presence of AAF without associated thrombotic events, i.e. the diagnosis of APS cannot be established and yet a greater tendency to develop SLE-PNL is observed in this group of patients. It is not known if the neurological manifestations could be conditioned by subclinical thrombotic events or if the PSA by an alternative mechanism produce lesions in the nervous system structures.
Cognitive impairment in SLE-NP: Among the manifestations of SLE-NP, cognitive impairment stands out as a frequent manifestation that compromises the quality of life of patients with SLE (35). The ACR defines cognitive deficit as a deficit in one or more of the following domains of cognition: simple attention, complex attention, memory, visual processing, language, reasoning and problem solving, and executive function.36
Table 2 describes the prevalences of cognitive impairment recorded in selected studies using different neuropsychological tests to establish the presence of cognitive deficits.37–47
|
Study |
Neuropsychological testing |
Number of patients |
Prevalence of cognitive deficits |
|
Ainiala H, et al.37 |
46 |
80% |
|
|
Brey RL, et al.38 |
ANAM |
128 |
79% |
|
Hollyday SL, et al.39 |
ANAM |
67 |
79% |
|
Sanna G, et al.40 |
323 |
11% |
|
|
Kozora et al.41 |
NRS |
67 |
21% |
|
Hanly JG, et al. 42 |
ANAM |
68 |
50% |
|
Adhikari et al.43 |
MoCA |
44 |
25% |
|
Julian et al.44 |
PDQ |
138 |
27% |
|
Conti F, et al.45 |
GCDs |
58 |
31% |
|
Calderon J, et al.46 |
CANTAB |
82 |
20% |
|
Pradhan V, et al.47 |
AMAN |
60 |
36% |
Table 2 Studies reporting the prevalence of cognitive impairment in SLE patients in the last 15 years.
ANAM, automated neuropsychological assessment metrics; CANTAB, cambridge neuropsychological test automated battery; CSI, cognitive symptom inventory; SLAM, systemic lupus activity measure; GCDs, global cognitive dysfunction score; Formal NP testing, Representative neuropsychological test battery derived from the ACR MoCA, montreal cognitive assessment; NRS, scripps neurologic rating scale; PDQ, perceived deficits questionnaire
Cognitive impairment in SLE does not necessarily develop parallel to the disease. There is no linear relationship between serum antibody levels and CNS pathological findings. Neuronal damage and cell loss occurs in a non-inflammatory setting, consistent with clinical data that cognitive impairment may be progressive in the absence of vasculitis or thrombosis.
Regarding the evaluation of cognitive functions in patients with SLE, it is recommended that the neuropsychological assessment should last approximately one hour, evaluating simple and complex attention, memory, visual-spatial processing, language, reasoning, problem solving, psychomotor speed and executive functions (Table 3).36,48
|
Test |
Cognitive function assessed |
|
Wechsler Adult Intelligence Scale |
Psychomotor speed, concentration, working memory |
|
Digit and symbol substitution test |
Psychomotor speed, concentration, grapho-motor skills |
|
Trail Making Test - Part B (Trail Making Test) |
Psychomotor speed, attention, cognitive sequencing |
|
Stroop color and word test (Stroop test) |
Complex attention with semantic interference |
|
California Verbal Learning Test |
Learning and recall of verbal material |
|
King-Osterrieth Complex Figure Test |
Learning and visual memory |
|
COWAT Test (Controlled Oral Word Association Test) |
Phonetic and semantic verbal fluency |
|
Finger tapping test (Finger tapping test) |
Simple speed measurement and motor control |
|
Criteria for cognitive impairment: |
|
|
Cognitive impairment: 2 or more standard deviations below the mean |
|
|
Focal cognitive impairment: 1 or more measures of a domain with impairment. Multifocal (diffuse) cognitive impairment: 2 or more domains affected |
|
Table 3 Neuropsychological tests recommended to assess the presence of cognitive impairment in patients with SLE
Transcranial doppler
Although Doppler technology originated in 1842 with the description of the Doppler effect by the Austrian physicist and mathematician Christian Andreas Doppler, it was not until the middle of the 20th century that it began its application in the medical field with carotid Doppler. However, it was not until 1982 when R. Aaslid et al. introduced transcranial Doppler (TCD) to evaluate intracranial circulation.49 Since then TCD has gained a place among the methods of established value to study the state of cerebral circulation, not only because it is a noninvasive and easily reproducible technology, but also because it provides additional information about cerebral circulation that is not possible to obtain by other types of cabinet studies.50,51
Transcranial doppler method and technique: The DTC allows the measurement of various physiological parameters of blood flow velocities (BFV) in the main basal arteries of the polygon of Willis through a low frequency transducer (2MHz) that is able to penetrate the cranial bony structures. Through established bone "windows" it is possible to obtain the ultrasonographic recording of the different basal arteries. A spectral image of the cerebral blood flow velocities during the cardiac cycle is obtained, and from this image it is possible to determine the systolic and diastolic velocities, which are essential to calculate the mean velocity, as well as the pulsatility and vascular resistance indexes, and thus establish the normality or abnormality of cerebral circulation (Figure 2).52
|
Formulas to be calculated using Doppler Spectra |
||
|
Speed media |
Index of pulsatility |
Index of resistance |
|
(VS-VD/3)VD |
(VS-VD)/VM |
(VS-VD)/VS |
Diastolic flow velocity (DV) and description of the formulas for calculating physiological parameters of cerebrovascular hemodynamics.
Acoustic bone "windows" are defined as the bony portions of the skull that present the least resistance to the passage of sound emitted by the low-frequency ultrasound transducer. Three bony "windows" have been described for analyzing cerebral arterial blood flow: transtemporal, transorbital and suboccipital. The transtemporal window above the zygomatic arch allows assessment of the VFS in the internal carotid artery (supraclinoid portion), middle cerebral artery (segments M1 and M2), anterior cerebral artery (segment A1) and posterior cerebral artery (segments P1 and P2). The transorbital window allows evaluation of the ophthalmic artery and the internal carotid artery (carotid siphon) and through the suboccipital approach ultrasonic signals of the vertebral arteries and the basilar artery are obtained (Figure 3).52,53
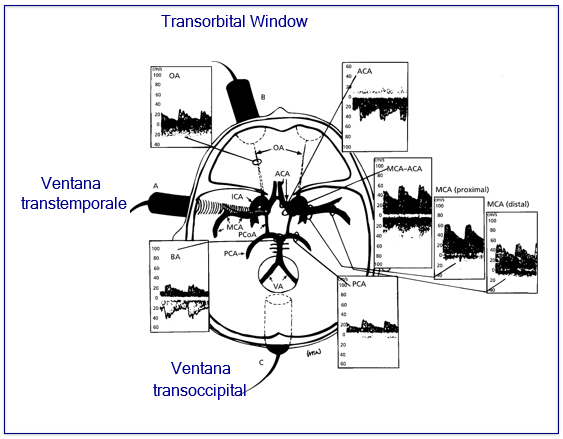
Figure 3 Spectra recorded through the different acoustic windows and corresponding intracranial arteries. ACA, anterior cerebral A; OA, ophthalmic A; MCA, middle cerebral A; MCA/ACA, internal carotid bifurcation in middle and anterior cerebral; PCA, posterior cerebral A; BA, basilar A; VA, vertebral A
Transcranial Doppler clinical applications, advantages and limitations: Table 4 lists the main applications of transcranial Doppler technology, as well as its advantages and limitations.
|
Main indications of transcranial doppler |
|
Detection and monitoring of cerebral vasospasm. |
|
Detection of stenosis and occlusion of intracranial arteries. |
|
Monitoring in the neurosurgical intensive care unit. |
|
Assessment of cerebral circulatory arrest in cases of encephalic death |
|
Detection of cerebral microemboli |
|
Cerebral vasomotor reactivity test |
|
Reperfusion monitoring during intravenous thrombolysis |
|
Evaluation of cerebral circulation in patients with acute cerebral infarction. |
|
Diagnosis of right-to-left shunts (e.g., patent foramen ovale) |
|
Study of cerebral hemodynamic reserve and collateral flow. |
|
Advantages of the use of transcranial doppler |
|
Non-invasive, fast and reproducible method |
|
Allows real-time assessment of cerebral circulation status |
|
The equipment is portable and the study can be performed at the patient's bedside. |
|
Less costly than other diagnostic techniques that assess cerebral circulation |
|
No need to use radiocontrast substances (no allergic reactions). |
|
Limitations of transcranial doppler use |
|
Dependent operator. Special training required |
|
Deficient bone window: In 10% of normal individuals it is not possible to obtain Doppler signals through the transtemporal "ultrasonic window". |
|
Anatomical variations of the polygon of Willis sometimes prevent proper evaluation. |
Assessment of cerebrovascular reactivity by DTC: Vasomotor reactivity can also be assessed by TCD by determining the changes in VFS in response to acetazolamide injection, hyperventilation, CO inhalation2 or by apnea testing that promotes CO retention2.55–57 As shown in Figure 4, the previous maneuvers produce changes in the distal circulation or microcirculation such that, as a consequence of hyperventilation-induced hypocapnia, vasoconstriction occurs, while hypercapnia induced by CO inhalation2 or acetazolamide application produces vasodilatation. These changes are easily perceived when recording blood flow velocities in the middle cerebral artery; vasoconstriction is manifested by decreased velocities and vasodilatation by increased velocities. The above is a way to evaluate the so-called cerebral autoregulation and above all it is considered that the alteration of the cerebrovascular reactivity is associated with endothelial dysfunction of the cerebral microcirculation.58
The evaluation of cerebrovascular reactivity is of particular importance to estimate the degree of cerebral hemodynamic impairment when there is significant occlusive disease of the carotid arteries and poor collateral flow. This test allows identification of patients at increased risk of cerebral hypoperfusion. When cerebrovascular reactivity is decreased, it indicates that the reserve capacity of cerebral autoregulation is also reduced and correlates with risk of recurrent cerebral ischemia in patients with significant stenosis or occlusion of the extracranial internal carotid artery.59,60
In recent years it has been shown that altered cerebrovascular reactivity correlates with cerebral small vessel involvement, in the form of microangiopathy usually as a consequence of arteriolosclerosis. Thus, it is considered that, in the absence of occlusive disease of the large cerebral arteries (such as extracranial carotid arteries), the presence of reduced cerebrovascular reactivity is equivalent to intracranial small vessel vasculopathy and is usually associated with increased resistance and pulsatility indices by increasing the stiffness of the arteriolar walls of the cerebral microcirculation.60–65
The most accurate way to evaluate cerebrovascular reactivity is through CO2 inhalation, due to the control of the CO2 concentration administered to the patient, besides being a non-invasive, easy and quick method to perform. The test consists of a controlled inhalation of a CO2-enriched gas mixture, for example carbogen gas (5% CO2, 95%O2). The latter allows a volume percent increase of CO2 in the airflow of the subject under study (Figure 5). A volume percent and the partial pressure of CO2 do not correlate directly, since the latter depends on atmospheric pressure and altitude at sea level. However, the above estimate can be applied as follows: the mean reported flow increment per volume percent is 23±5%. As a clinical cut-off point, it is recommended to use the mean -2 standard deviations = approximately 10%. Flow increase below 10% can therefore be considered as altered cerebrovascular reactivity.50
On the left middle cerebral artery Doppler spectrum by TCD in a healthy subject shows the changes in VFS according to end-expiratory CO2.
Evaluation of cerebral circulation by TCD in SLE patients
There are previous reports on the evaluation of cerebral circulation using TCD in patients with SLE, most of them focused on the detection of microembolic signals.66–69 One of the applications of TCD is the ability to detect microembolic signals (MES), which correspond to microemboli that can be platelet, fibrinogen, fatty or atheromatous material that appear on the blood flow in intracranial arteries.
In one of the first studies performed with the aim of finding an association between antiphospholipid antibodies, MES and cerebrovascular ischemic manifestations, Rademacher et al.66 evaluated 70 patients with SLE, 25 of them with a history of cerebral ischemia, of whom the presence of MES was documented in 15 (60%). Myecrememboli were also found in 15 of 38 patients with a diagnosis of secondary antiphospholipid syndrome (39%). The authors conclude that the presence of MES may be a marker of disease activity in patients with SLE and APS.
On the other hand, only one study has focused on the evaluation of cerebral blood flow velocities and cerebrovascular reactivity in SLE patients. Tunde et al (70) found that mean cerebral artery velocities in SLE patients without neurological symptoms and with normal MRI (n=9) were higher than in healthy controls (n=10), 74.5±3.1 cm/sec vs. 56±2.8 cm/sec, respectively, while patients with focal neurological symptoms (n=6) had lower FSC velocities in the affected hemisphere than healthy controls (44.5±2.6 cm/sec). After the application of IV acetozolamide (1 g) to assess cerebrovascular reactivity, the magnitude of the response was greater in patients with SLE without NP manifestations (125±4.6 cm/sec) than in healthy controls (87.3± 4.1 cm/sec) and patients with SLE with focal neurological manifestations (66.5±3.1 cm/sec); it is necessary to clarify that the authors do not indicate the time of evolution of the neurological symptoms of these patients. The authors mention that "the increase in resting MCA FSC velocities suggests an alteration in cerebral vessels (edema?, inflammation?) that is detectable in early stages of SLE with TCD, before neurological symptoms appear" and argue that in patients with low FSC velocities the vascular damage has evolved to more advanced stages with endothelial proliferation, mural thickening and mural deposits of platelets, in addition to neurological symptoms.70
Early and timely detection and treatment of cognitive impairment in SLE patients should improve their quality of life. Studies carried out with the aim of investigating the effect that cognitive alterations can have on the daily functioning of patients, specifically, on work occupation, show that patients with more severe memory impairment have a higher prevalence of unemployment and limitations in their daily activities, especially considering that the spectrum of patients with this disease are young women. The prevalence of this neuropsychiatric disorder is unknown in our population, so it is essential to know the magnitude of this neurological manifestation in our environment and to provide timely prevention and treatment measures.
The pathogenic factors associated with and triggering cognitive impairment in SLE are still unknown and it is very likely that it is multifactorial; essentially the following lines of research are being pursued (Figure 1): that it is a consequence of the production of autoantibodies directed at components of brain tissue, that inflammatory mechanisms are involved by cytokines in the presence of damage to the blood-brain barrier, or that cerebrovascular alterations associated mainly with microangiopathic processes participate. Although most studies are focused on demonstrating the autoimmune mechanism, there is evidence that allows us to support the participation of the vascular mechanism and currently there is transcranial Doppler technology that allows us the noninvasive evaluation of cerebral circulation in an easy, fast and economically accessible way.
Therefore, it is essential to increase our knowledge of the pathogenic mechanisms responsible for cognitive alterations in SLE, so it is amply justified to carry out a research project that evaluates the state of the cerebral circulation in patients with SLE and to determine its potential involvement in the development of cognitive impairment associated with systemic lupus erythematosus.
Based on the above, the general research question of our study can be stated as follows:
Do SLE patients with cognitive impairment have alterations in cerebral circulation determined noninvasively by transcranial Doppler?
The specific research questions are as follows:
What is the prevalence of cognitive impairment in patients with SLE in our setting?
What is the frequency and magnitude of cerebral circulation alterations determined by TCD in SLE patients compared to healthy controls?
Do patients with SLE and cognitive impairment have greater alterations in cerebral circulation determined by TCD compared to patients with SLE without intellectual impairment?
Patients with SLE and cognitive impairment present alterations in cerebral circulation manifested by increased cerebral blood flow velocities and decreased cerebrovascular reactivity (CVR), as assessed by transcranial Doppler technology.
General objective
To determine the frequency and magnitude of cerebral circulation alterations (blood flow velocities, pulsatility and cerebrovascular reactivity) measured by TCD in patients with systemic lupus erythematosus and cognitive impairment, compared with patients with SLE without intellectual impairment and with healthy control subjects.
Specific objectives
Design
Type of Research
Cases and controls nested to a prospective cohort, with the following characteristics.
Observational (X) Experimental ( )
Longitudinal ( ) Transversal (X)
Prospective (X) Retrospective ( )
Descriptive ( ) Comparison (X)
Open ( ) Blinded (X)
Study groups
Problem group: Patients with SLE and cognitive impairment belonging to a cohort of 100 patients from the Department of Immunology and Rheumatology of INCMNSZ, cohort that is part of the protocol "Predictors of cognitive dysfunction in patients with generalized lupus erythematosus". These patients were selected if they met four or more criteria according to the ACR for SLE, were identified and started follow-up within the first 12 months of SLE diagnosis. To date, some have been followed for up to 8 years. Cognitive impairment was documented by comprehensive neuropsychological testing.
Witness Groups Control group 1: Patients with Systemic Lupus Erythematosus without evidence of cognitive impairment according to evaluation with neuropsychological tests, belonging to the same cohort as the cases.
Control group 2
Apparently healthy control subjects. Because about 90% of SLE patients are younger than 50 years of age and 90% are female, this control group was oriented to select young women.
Sample size calculation
The formula for comparison of two means (between the SLE and cognitive impairment group and the SLE group without impairment) was used:
n = 2 (Zα + Zβ)2 S2 2 (1.96 + 0.84)2 (5)2 392 = 28
d2 (3.75)2 14
where:
n = number of subjects needed in each of the groups.
Zα = 0.05 → 1.96
Zβ = 0.20 → 0.84
S2 = variance of the quantitative variable having reference group → 5%.
d = minimum value of the difference to be detected = 15% [considering that the mean CVR in the control group is 25, then (15% x 25) = 3.75].
Therefore, the n will be 28 patients per group + 10% expected losses, mainly due to technical problems of the DTC.
Selection criteria
Inclusion criteria
Exclusion criteria
Elimination criteria
Procedures
In patients with SLE who agreed to participate in the study, the following procedures were performed:
Structured clinical interview and neurological evaluation as well as review of the clinical record, collecting the following information:
Performance of neuropsychological tests recommended by the American Academy of Rheumatology to assess the presence of cognitive impairment in patients with SLE.
The tests include the evaluation of simple and complex attention, memory, visual spatial processing, language, reasoning, problem solving, psychomotor speed and executive functions, through the application of the following tests:
Transcranial Doppler evaluation of cerebral circulation
The evaluation of cerebral circulation was carried out by obtaining recordings of the middle cerebral arteries using Nicolet Pioner equipment. The following measurements were collected:
The RCV or cerebral vasomotor reactivity to hypercapnia was evaluated by inhalation of a mixture of CO2 at 8%, O2 at 21% and nitrogen balance for one minute -administered through a mask-, and was obtained by determining the percentage increase in mean velocity (MV) flow that occurred during the test in relation to the baseline mean velocity, using the following formula:
RCV = MV at end of inhalation - basal MV / basal MV X 100%.
In each patient, 3 evaluations were performed. After each evaluation, the return to baseline flow velocity values was documented. The reactivity values included in the analysis are the average of 3 tests.
In healthy control subjects, demographic data and measurements obtained from TCD evaluation of cerebral circulation were collected. Intellectual status was assumed to be normal.
Variables
Dependent variable: The dependent variable is the presence or not of cognitive impairment. Cognitive impairment was considered to be present if one of the neuropsychological tests evaluated was 2 or more standard deviations below the mean. This is considered a dichotomous variable in which there is or is not cognitive impairment. However, it was also classified into mild, moderate and severe impairment depending on the magnitude and number of altered tests.
Independent variables
These are the variables related to the hemodynamics of the cerebral circulation measured by TCD:
Independent covariates
These variables are those related to demographic data, to the characteristics of the lupus disease and those that may influence the cognitive functions of the patients.
Analysis plan
Computer tool: The SPSS program was used.
Descriptive statistics: The description of the data was carried out in terms of measures of central tendency and dispersion, for the numerical variables, as well as percentages for the nominal variables. Descriptive analysis of the group under study was performed, in relation to the main demographic and clinical characteristics of SLE patients and the prevalence of cognitive impairment in this SLE cohort was determined.
Inferential analysis: The following comparative analyses of cerebral circulation hemodynamic variables obtained by TCD were performed:
For statistical analysis, Student's t-test was used to compare the groups of SLE patients and healthy controls and to compare the groups of SLE patients with and without cognitive impairment. To compare the results including the healthy control group, the degree of cognitive impairment and the time of disease evolution, the ANOVA test was used. Finally, multivariate analysis was performed by binary logistic regression to assess the independent relationship of cerebrovascular hemodynamic variables with cognitive impairment adjusting for variables that could influence impairment; the Hosmer-Lemeshow test was used for goodness-of-fit. All p values are two-tailed and it was considered significant if the p value is less than 0.05.
Clinical description of SLE patients included in the study
Sixty-seven SLE patients from the INCMNSZ SLE cohort were studied, including 61 females (91%) and 6 males (9%), the mean age being 33.9±7.4 years. Table 5 shows the main demographic and clinical characteristics of the 67 patients. They were young patients at the time of diagnosis (average age 23 years, in addition to having a very short interval from the onset of the clinical picture to the definitive diagnosis (8 months); however, at the time of carrying out the present study the cohort consisted of patients with a chronic course of 11 years on average from the onset of the disease and with numerous relapses (almost half of the patients had more than 3 relapses).
|
Age, mean±SD, years |
33.9±7.4 |
|
|
Sex, (%) |
Women |
61 (91.0) |
|
Men |
6 (9.0) |
|
|
Age at diagnosis, mean±SD, years |
23.1±6.8 |
|
|
Time evolution from symptom onset to diagnosis, mean±SD, months |
8.3±17.9 |
|
|
Time of disease progression, mean±SD, years |
9.8±4.5 |
|
|
SLE criteria present at |
Mucocutaneous |
50 (74.6) |
|
time of diagnosis of the |
Articulate |
61 (91.0) |
|
disease (%) |
Renal Photosensitivity |
31 (46.3) |
|
Lupus discoid |
21 (31.3) |
|
|
Seizures |
6 (9.0) |
|
|
Psychosis |
6 (9.0) |
|
|
Hematological |
2 (3.0) |
|
|
Serositis |
24 (35.8) |
|
|
Immunological |
22 (32.8) |
|
|
Antinuclear antibodies |
42 (62.7) |
|
|
48 (71.6) |
||
|
Relapse by activity (%) |
No |
4 (6.0) |
|
1-3 |
34 (50.1) |
|
|
> 3 |
29 (43.9) |
|
|
Reason for relapse (%) |
Renal |
31 (45.3) |
|
Articulate |
33 (49.3) |
|
|
Mucocutaneous |
27 (40.3) |
|
|
Serositis |
14 (20.9) |
|
|
Hematological |
23 (34.3) |
|
|
Neurological manifestations* |
Seizures |
8 (11.9) |
|
Myelitis |
3 (4.5) |
|
|
Headache |
18 (26.9) |
|
|
Neuropathy |
10 (14.9) |
|
|
Cerebrovascular disease |
7 (10.4) |
|
|
Korea |
4 (6.0) |
|
|
Tremor |
2 (3.0) |
|
|
Vascular risk factors (%) |
Arterial hypertension |
14 (20.9) |
|
Diabetes mellitus |
2 (3.0) |
|
|
Previous smoking |
25 (37.3) |
|
|
Alcoholism |
6 (9.0) |
|
|
Dyslipidemia |
24 (35.8) |
|
|
Hypothyroidism |
10 (14.9) |
|
|
Other manifestations of SLE (%) |
Antiphospholipid syndrome |
22 (32.8) |
|
Venous thromboembolism |
7 (9.0) |
|
|
Medications used (%) |
Prednisone (%) |
66 (98.5) |
|
Azathioprine (%) |
52 (77.6) |
|
|
Chloroquine (%) |
40 (59.7) |
|
|
Cyclophosphamide (%) |
26 (37.3) |
|
|
Mofetil (%) |
15 (22.4) |
|
|
Methotrexate (%) |
8 (11.9) |
Table 5A Demographic and clinical characteristics of the 67 patients with SLE
Up to 50% of the patients had presented some neuropsychiatric manifestation, the main ones being headache, peripheral neuropathy, seizures and cerebral vascular disease. The predominant vascular risk factors in our cohort were smoking, dyslipidemia and hypertension. The most common treatment, as expected, was prednisone, the average dose being 18±12 mg per day.
Prevalence of cognitive impairment in patients with SLE
Of the 67 patients with LEG, alterations in the evaluation of cognitive functions were found in 29 patients (43.3%), which were classified as mild in 16 patients (23.9%), moderate in 6 (9.0%) and severe in 7 patients (10.4%). Table 5 shows the distribution of demographic data, clinical manifestations and vascular risk factors, according to cognitive impairment. Statistical significance was found for cognitive impairment only for the vascular risk factors arterial hypertension (p=.03) and venous thromboembolism (p=.04).
|
Normal n=38 |
Slight n=16 |
Mod/severo n=13 |
p |
|
|
Sex, (%) Male |
4 (10.5) |
0 (0) |
2 (15.4) |
|
|
Woman |
34 (89.5) |
16 (100.0) |
11 (84.6) |
0.3 |
|
Age, mean±SD, years |
33.1±6.3 |
33.1±7.6 |
33.7±9.4 |
0.47 |
|
Age at diagnosis, mean±SD, years |
22.2±6.4 |
23.6±7.9 |
24.9±6.9 |
0.4 |
|
SLE evolution time, mean±SD, years |
10.7±4.0 |
10.2±4.9 |
12.5±5.4 |
0.46 |
|
Relapses, mean±SD |
2.8±2.3 |
4.1±3.2 |
3.5±2.1 |
0.23 |
|
Vascular risk factors (%) |
||||
|
Arterial hypertension |
9 (23.7) |
0 |
5 (38.5) |
0.03 |
|
Diabetes mellitus |
0 |
1 (6.2) |
1 (7.7) |
0.25 |
|
Smoking |
15 (39.5) |
3 (18.8) |
7 (53.8) |
0.13 |
|
Dyslipidemia |
14 (36.8) |
6 (37.5) |
4 (30.8) |
0.91 |
|
Etilism |
4 (10.5) |
0 |
2 (15.4) |
0.3 |
|
Obesity |
10 (26.3) |
4 (25.0) |
4 (30.8 |
0.93 |
|
Venous thromboembolism |
1 (2.6) |
3 (18.8) |
0 |
0.04 |
|
SLE inclusion criteria (%) |
||||
|
Mucocutaneous |
26 (68.4) |
12 (75.0) |
12 (92.3) |
0.23 |
|
Joint disease |
36 (94.7) |
13 (81.2) |
12 (92.3) |
0.28 |
|
Seizures/Psychosis |
3 (7.9) |
3 (18.8) |
0 |
0.2 |
|
Renal disease |
16 (42.1) |
7 (43.8) |
8 (61.5) |
0.46 |
|
Hematological |
12 (31.6) |
6 (37.5) |
6 (46.2) |
0.63 |
|
Serositis |
12 (31.6) |
5 (31.2) |
5 (38.5) |
0.89 |
|
Photosensitivity |
12 (31.6) |
5 (31.2) |
13 (100) |
0.99 |
|
Lupus discoid |
4 (10.5) |
1 (6.1) |
1 (7.7) |
0.86 |
|
Immunological |
26 (68.4) |
7 (43.8) |
9 (69.2) |
0.19 |
|
Antinuclear antibodies |
28 (73.7) |
11 (68.8) |
9 (69.2) |
0.91 |
|
Neurological Manifestations |
21 (55.3) |
7 (43.8) |
6 (46.2) |
0.69 |
|
Seizures |
4 (10.5) |
2 (12.5) |
2 (15.4) |
0.89 |
|
Cerebrovascular disease |
3 (7.9) |
2 (12.5) |
1 (7.7) |
0.85 |
|
Headache |
12 (31.6) |
3 (18.8) |
3 (23.1) |
0.58 |
|
Polyneuropathy |
6 (15.8) |
2 (12.5) |
2 (15.4) |
0.95 |
Table 5B Demographic data, clinical manifestations, and vascular risk factors according to cognitive impairment in the 67 patients with SLE
Evaluation of cerebral circulation hemodynamics determined by transcranial Doppler in patients with SLE compared to healthy controls
Twenty-nine healthy control subjects were included including 24 females (83%) and 5 males (17%), the mean age being 35.2 years (SD±11.7 years). There were no differences in age and sex between control subjects and SLE patients. Table 6 shows the FSC velocities, pulsatility index and middle cerebral artery cerebrovascular reactivity in the 29 control subjects and the 67 SLE patients.
|
Control Group n= 29 |
LES Group n = 67 |
p |
|
|
Peak systolic velocity |
105.9±17.1 |
118.5±23.3 |
0.01 |
|
End-diastolic velocity |
47.0±8.3 |
56.3±15.3 |
0.003 |
|
Average speed |
66.6±9.8 |
77.0±17.5 |
0.004 |
|
Pulsatility index |
0.86±0.13 |
0.82±0.14 |
0.16 |
|
Cerebrovascular reactivity |
32.3±15.8 |
19.5±18.0 |
0.001 |
Table 6 Comparison of hemodynamic parameters of cerebral circulation, between the control group of healthy subjects and the group of SLE patients
All cerebrovascular hemodynamic parameters measured showed statistical significance between the control group of healthy subjects and the SLE group, except pulsatility index (p=.16). Cerebrovascular reactivity showed the highest significant difference (p=.001) between healthy subjects and SLE patients. When evaluating the effect of the time of evolution of lupus disease with the deterioration of cerebrovascular reactivity and it was evident that the longer the time of evolution the greater the alteration of CVR (Figure 6). Compared with the average increase in blood flow velocity in response to hypercapnia in healthy control subjects (CVR= 32.3%), in SLE patients with less than 10 years of evolution the CVR was 20.5%, remained similar between 10 and 14 years of evolution and was markedly reduced in SLE patients with 15 or more years of evolution (CVR = 11.3%).
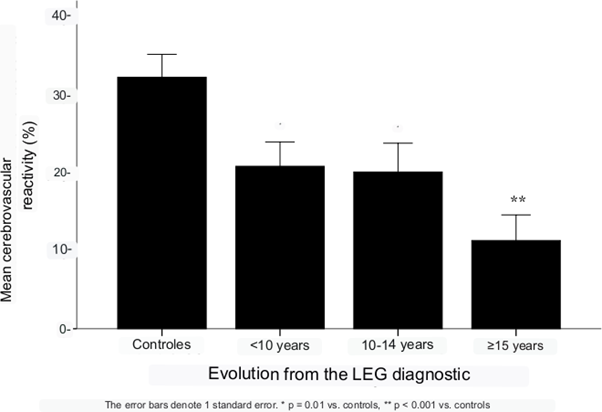
Figure 6 Cerebrovascular reactivity in healthy controls (n = 29) and in patients with SLE according to time since diagnosis (n = 67).
Evaluation of cerebral circulation hemodynamics determined by transcranial Doppler in SLE patients according to the presence of cognitive impairment and healthy control subjects
Table 7 shows cerebral blood flow velocities, pulsatility index, and middle cerebral artery cerebrovascular reactivity among the 38 patients with SLE and cognitive impairment and the 28 patients with SLE and normal neuropsychological evaluation.
|
LEG without DC n= 38 |
LEG with DC n = 29 |
p |
|
|
Peak systolic velocity |
117.7±21.4 |
119.6±25.9 |
0.75 |
|
End-diastolic velocity |
56.5±13.2 |
56.1±17.8 |
0.97 |
|
Average speed |
76.9±15.7 |
77.2±19.9 |
0.95 |
|
Pulsatility index |
0.80±0.11 |
0.84±0.15 |
0.25 |
|
Cerebrovascular reactivity |
24.4±18.2 |
13.1±15.9 |
0.01 |
Table 7 Comparison of hemodynamic parameters of cerebral circulation, among patients with SLE according to the presence of cognitive impairment
While snguine flow velocities and pulsatility index showed no differences, significant alteration of cerebral vasomotor reactivity was found in patients with SLE and cognitive impairment (increase of only 13.1±15.9% to hypercapnia, compared to increase of 24.2±18.2% in patients without cognitive impairment; p=.01). When including in the analysis the group of healthy control subjects and stratifying the SLE patients according to cognitive performance in the neuropsychological tests, a gradient was found in the degree of alteration of cerebral vasomotor reactivity with greater deterioration of intellectual functions, such that the mean CVR±SD of increase to hypercapnea in the healthy control group was 32.6 ±16.5%, compared with 24.7±18.3%, 14.3±15.3% and 8.3±12.4 in patients with SLE and normal cognition, mild and moderate/severe impairment, respectively (Figure 7).
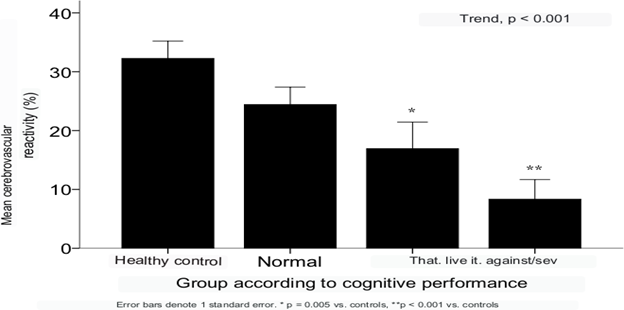
Figure 7 Cerebrovascular reactivity in healthy controls (n = 29) and in patients with LEG according to their cognitive performance (n = 67).
Finally, when adjusting for cerebrovascular reactivity through multivariate analysis by binary logistic regression with other independent factors related to cognitive impairment, CVR was found to remain significant reaching the association estimate odds ratios of 9.8 (95% CI 2.9 - 32.8; p<0.001) (Figure 8).
Patients with SLE present various neuropsychiatric clinical manifestations, with cognitive impairment being one of the most frequent as demonstrated in the present study reaching a prevalence of 43%, reaching a moderate to severe degree in 20% of the cases. The main finding of our study is the high frequency of alterations in the parameters of cerebrovascular hemodynamics in patients with SLE, which are more noticeable in patients with cognitive impairment. Of the hemodynamic parameters of the cerebral circulation evaluated, cerebrovascular reactivity was found to be particularly altered in patients with SLE and cognitive impairment, and a gradient of abnormality was observed with the degree of impairment of intellectual functions (Figure 7). This suggests that vascular damage mechanisms in the cerebral microcirculation participate in the development of the cognitive impairment observed in SLE patients.
Small vessel vasculopathy is considered the most frequent structural alteration in postmortem brain investigations of patients with LEG, characteristic histological findings include intimal cell proliferation, increased fibrous tissue and mucosal hyperplasia, the vascular lumen may be occluded by fibrin and thrombus, damaged vessels may be surrounded by activated microglia and inflammatory infiltrates and small infarcts with areas of necrosis. It is important to note that noninflammatory vasculopathy has been reported in pathologic studies more frequently than inflammatory vasculopathy. We believe that the alterations in the elevated flow velocities found in the middle cerebral arteries of our patients support the presence of the vascular damage described previously.
Thus, acute vasculopathy may induce a transient increase in cerebral blood flow velocities that can be observed in TCD. These vasculopathic changes could represent a wide variety of clinical manifestations ranging from asymptomatic microinfarcts in the deep white matter in patients with apparently quiescent LEG or mild systemic activity, to severe central nervous system manifestations (delirium, seizures, etc) with significant systemic activity and elevated SLEDAI indices. It is very likely that the pathogenesis of cognitive impairment in SLE is multifactorial. A recent study on the pathophysiology of NPLES demonstrates increased metabolic activity in the hippocampus or amygdala after disruption of the blood-brain barrier. Mice expressing anti-DNRA antibodies exhibit a pattern of metabolic changes that is not present in DNRA-negative mice. Local metabolic activity is relatively reduced at 2 weeks after blood-brain barrier disruption. Thus the reduced metabolic activity is transient and then a consistent change is unlikely to be detected in human cross-sectional studies. There is also anti-DNRA antibody-mediated neuronal loss in the hippocampal formation within the first week of anti-DNRA antibody exposure. However, the inverse correlation in anti-DNRA mice between hippopampal cell number and metabolic activity at 4 weeks suggests the gradual development of a distinct delayed tissue response. Blood-brain barrier integrity is restored early after lipopolysaccharide administration. There is no detectable antibody in the hippocampus days after blood-brain barrier disruption. Thus, the increase in metabolism that occurs 2 to 4 weeks after LPS administration reflects a compensatory increase in metabolism that is either a "normalized tissue activity" or a non- neuronal inflammatory mechanism (e.g., a glial inflammatory response to neuronal necrosis) that increases metabolic activity after the transient reduction at 2 weeks. It would be interesting to know if the increase continues after 4 weeks allowing higher than normal hippocampal metabolism in anti DNRA mice. Further study of microglial activity in anti- DNRA mice will be required. In contrast, in animals without anti-DNRA, cell count and metabolism in the hippocampal formation exhibit a positive correlation consistent with a localized inflammatory glial response that does not alter memory. Thus, fluorodeoxyglucose metabolism may be normal in both healthy and diseased tissues, and similar metabolic profiles may be associated with different behavioral outcomes. This phenomenon of similar FDG PET findings, with different etiologies and behavioral outcomes, was also observed in anti-DNRA mice with blood-brain barrier disruption in the amygdala.
The prevalence of cognitive impairment in this SLE cohort was 43%. There are several potential causes of cognitive dysfunction in SLE patients. In the present study we demonstrated that SLE patients have a high proportion of alterations in cerebral circulation mainly due to elevated cerebral blood flow velocities and in patients with cognitive
impairment we found a significant alteration of cerebrovascular reactivity, suggesting that vascular mechanisms that damage cerebral microcirculation are involved in the development of cognitive impairment in SLE patients.
None.
The authors declare no conflicts of interest.

©2023 Carrillo. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.