Journal of
eISSN: 2373-6410


Case Report Volume 10 Issue 3
1Department of Neurosurgery, Manipal Hospital Whitefield, India
2Department of Radiology, Manipal Hospital Whitefield, India
Correspondence: Ganapathy S, Associate Consultant Neurosurgeon, Department of Neurosurgery, Manipal Hospital Whitefield, Bangalore, India
Received: April 09, 2020 | Published: May 15, 2020
Citation: Ganapathy S, Ullas A, Pandey P. Cervical spinal epidural arteriovenous fistulae presenting as compressive myelopathy. J Neurol Stroke. 2020;10(3):92-95. DOI: 10.15406/jnsk.2020.10.00417
Epidural Arterio-venous fistulae (EDAVF) are rare. They occur as abnormal Arterio-Venous or Capillary communications arising from major arteries of the neck or viscera and anastomose with intracranial venous sinuses or intradural spinal venous plexi, thereby short-circuiting the blood flow pathway and causing sudden and sustained venous hypertension. The retrograde flow results in venous bleeds, which may range from small petechiae on the cortical surface to major venous bleeds resulting in severe disability and even death. The shift of blood flow also can result in a steal phenomenon, serious enough to cause infarcts. Mechanical compression of the spinal cord remains thankfully the commonest symptom and is caused by the high flow fistulae leading to medullary and radicular symptoms, especially in conjunction with neurofibromatosis 1. Proper Identification and management depend on understanding the pathology, location and flow dynamics of the fistula. We present our experience with a large cervical epidural fistula with a review of literature and analysis of management protocols.
Keywords: epidural av fistula, compressive myelopathy, embolisation
Spinal epidural arteriovenous fistulae (EDAVF) are considered rare and difficult lesions to treat.1,2 The identification of such lesions was in doubt for many years. Rubenstein, Mourier and others considered them a subtype of dural AV fistulae. It was Spetzler who modified Oldfield’s classification adding a separate entry for EDAVFs.1–3 The latest classification by Takai and associates proposes a subdivision of EDAVFs into 2 types allowing management decisions to be simplified based on classification itself. 1–3 The progressive nature of the disease, overlapping clinical syndrome, and controversy regarding treatment make EDAVFs an interesting lesion to document and study.3 Over the last five years, reports of EDAVFs in literature have trebled, and interest is peaking. We add our experience to this increasing pool of interest and knowledge.
A 62-year-old male presented with right hemiparesis along with numbness and paresthesia over the right half of the body for 8 months. The symptoms began suddenly 8 months ago and gradually improved. His main complaint now was the spasticity along with increased frequency and hesitancy of urine. On examination at the outpatient clinic, he was found to have MRC (Medical Research Council) grade 4 power in his right upper and lower limb with spasticity of Ashworth grade 3, as well as decreased sensation over the right supraclavicular region and over the right shoulder. The patient was then investigated for the cause of his myelopathy with a Magnetic Resonance Imaging (MRI) of his cervical spine and brain. The MRI showed multiple flow voids epidurally, with a large draining vein at the craniovertebral junction entering the intradural space, compressing the cervical cord producing the resulting myelopathy (Figure 1A & B).
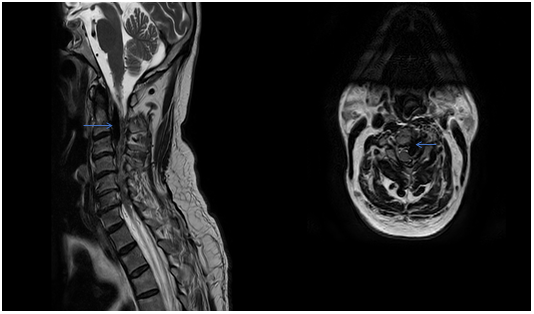
Figure 1A&1B MRI T2W images of the cervical spine showing the sagittal section of the compression of the cervical spinal cord secondary to large flow voids present inside and outside the dura.
The Axial image in figure 1b shows a large vessel compressing and displacing the cervical spinal cord with cord changes
.
A Contrast Computerized Tomography (CT) scan showed a large vascular malformation around the craniovertebral junction extending from C0 to C3 fed extradurally and extrevertebrally (Figure 2). The patient was explained the nature of the disease as well as the modalities of management. He was then taken up for a Digital Subtraction Angiographic study of the lesion with a concomitant glue/ onyx injection. The angiogram showed a large high cervical epidural AV fistula (EDAVF) being fed by branches from bilateral vertebral, ascending pharyngeal and deep cervical arteries, left more than right (Figure 3). The EDAVF however was exclusively present on the left side. The right sided feeders seemed to be miniscule and to contribute minimally to the flow. The patient was also seen to have a persistent Left Trigeminal artery which augmented flow from the left Internal carotid artery into the Left Vertebral artery. This presented us with an excellent setting for Endovascular treatment for the vertebral feeders.
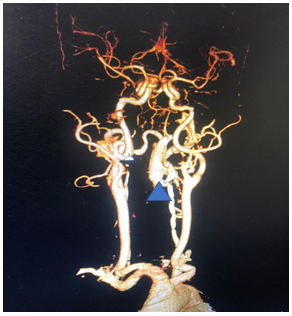
Figure 2 This shows the CT angiogram with 3D reconstruction of the sequences. The arrow shows the large EDAVF which would have considerably compressed the cord, being fed from the vertebral artery as well as deep cervical branches of the External Carotid system, making this a classic Extradural Arteriovenous Fistula.
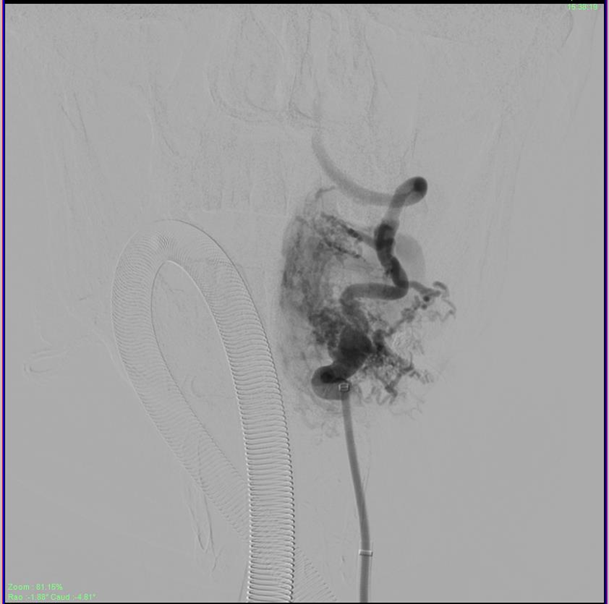
Figure 3 Microcatheter placed at the mouth of the feeder arising from the V2 segment of the ipsilateral vertebral artery.
The patient was then administered general anesthetic. IV heparin of approximately 1000U was administered every hour further on and a Heparin flush was connected to the femoral sheath. The 5F short sheath was then exchanged for a Neuron Max 088 Sheath which was placed under fluoroscopic guidance into the left proximal subclavian artery. A 5F Navien catheter was then advanced into the Left Vertebral Artery railroaded on a 035 guidewire. Over this the Neuron Max catheter was moved co-axially into the proximal vertebral artery as well. An Echelon Microcatheter was then taken over the 014 Traxcess microwire and feeders from the distal V2 segment of the vertebral artery were selectively catheterized and visualized. The large lateral feeder which was significantly dilated was chosen for onyx injection (Figure 4). DMSO was injected to fill the dead space of the microcatheter and Onyx was injected under multiple blank roadmap guidance into the feeder to fill the EDAVF sac partially. Reflux into the main vertebral artery was noted, after which the injection of the onyx was stopped. Post injection films showed complete obliteration of the feeders from the vertebral artery (Figure 5).
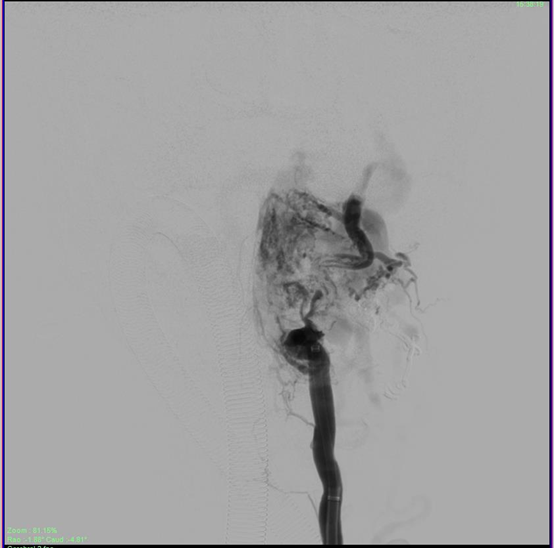
Figure 5 Partial obliteration of the EDAVF after onyx injection of the vertebral feeders, showing stasis in the draining veins.
The sheath was then repositioned into the left Costocervical trunk. (The Echelon microcatheter was then advanced distally into the branch over a microwire (Figure 6). Multiple vials of Onyx were injected to the fistula achieving complete obliteration of the venous sac in just over 55 minutes approximately. The Onyx was also seen filling the adjacent feeder branches as well obliterating all arterial feeders to the EDAVF. A check angiogram of bilateral subclavian arteries and the right vertebral artery showed considerable slowing of flow into the EDAVF with approximately 80-90% obliteration of the venous sac (Figure 7).
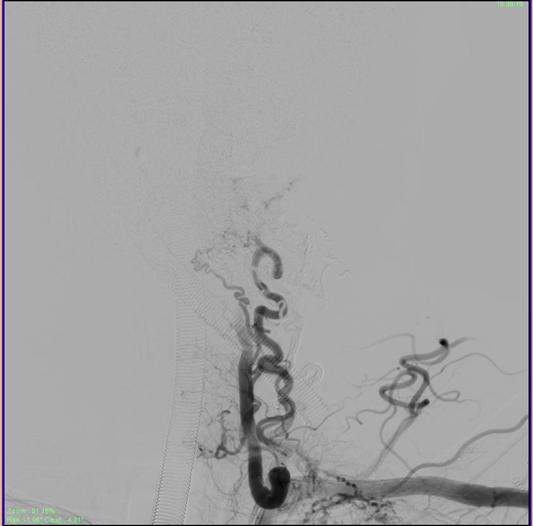
Figure 7 Check angiogram showing complete obliteration of the compressive venous sac post embolization, and stasis in the draining veins which will eventually empty and close.
The Persistent Trigeminal artery maintained good flow in the vertebral artery allowing for good filling of the basilar artery and its vital branches (Figure 8). The Persistent Trigeminal Artery connect the anterior circulation directly to the basilar artery. Hence, damage to 1 or more vertebral arteries will not reduce blood flow to the brainstem. Hence these patents have a high tolerance for vertebral artery manipulation and even thrombosis. The procedure was stopped, and the patient was extubated. Post op he was deficet free, alert conscious and content. The femoral sheath was removed the next day, and the patient was mobilized and discharged after 2 days. He has review in OPD after 3 months and repeat angiographic investigations have showed no filling in the EDAVF ever since.
The pathology and genesis of these peculiar and rare lesions is unclear. EDAVFs were considered by some as related to neurofibromatosis, injury or a pressure differential secondary to either surgery or other pathology of the spine.2,3 Venous thrombosis secondary to pathology or intervention may lead to development of these lesions as well.4 Compromised outflow lead to an abnormal reflux of blood which eventually finds its way into the perimedullary plexi, establishing the fistula, possibly the only aspect of etiopathogenesis agreed upon widely.3,4 Mechanical compression of the spinal cord are caused by the high flow fistulae leading to medullary and radicular symptoms, especially in conjunction with neurofibromatosis 1.
Another bone of contention is classification. Vascular pathology of the spine is broadly classified into AV Malformations and AV fistulae. (Ignoring vascular neoplasms and aneurysms which are even rarer).5 Attempts made by Di Chiro, Mournier, Rosenblum, Kendal, Yasargil, Cogen and Oldfield were subsequently ignored in place of newer descriptive classifications such as the ones proposed by Rodesh and Geibprasert in 2008.2–6 Another classification strategy is by Rangen-Castilla where the presence of a perimedullary draining vein isn’t important as long as clinical features of a myelopathy exist.4–6 The latest angiographic, and clinical classification has been proposed by Keisuke Takai (2018) where Extradural fistulae are added to the Rosenblum classification. They are further subdivided into subtypes based on the nature of the feeders and venous drainage (Table 1). The advantage of this classification is that treatment approaches can be decided based on the subtypes.2–5
Type |
Name |
Subtype |
Description |
1 |
Dural AVF |
As per the OLD Rosenblum Classification classically used |
|
2 |
Intramedullary Glomus AVM |
||
3 |
Intramedullary Juvenile AVM |
||
4 |
Perimedullary AVF |
A |
A single Feeder & Multiple small AVFs |
|
|
B |
Multiple Feeders & Medium Size AVFs |
|
|
C |
Multiple feeders & Giant AVFs |
5 |
Extradural AVF |
A |
With Intradural Draining Vein |
|
|
B |
Without Intradural Draining Vein |
Table 1 Takai classification of Dural and Epidural AV fistulae of the spine (2016).
Our case was aided by a very fortuitous occurrence of the primitive Trigeminal artery which was patent allowing us the manipulate the left vertebral artery without fear of consequences which may lead to hypoperfusion of the brainstem (spasm, dissection, rupture, or thrombosis). Post procedure flow was excellent allowing adequate perfusion of the brainstem throughout the procedure.
Management strategies range from surgical to endovascular. The deciding factor according to Takai and associates, is the presence of a perimedullary draining vein.6 Such a vein is amenable to surgical clipping allowing excellent resolution of compressive symptoms.6,7 The absence of a large vein, due to a complex venous drainage patterns, make surgery difficult, allowing Endovascular techniques to be superior. Here the nidus, was a high flow system, allowing the onyx more time to solidify and occlude the system. High flow systems are safer to manage surgically rather than low flow systems.7 The site of spinal involvement (level) also plays a pivotal role in deciding the mode of management. Craniovertebral lesions are safer to manage endovascularly rather than by open surgery.7,8 Here in our case despite there being a large draining vein (Type VA), we thought it prudent to deal with such a sensitive location through endovascular means. For endovascular treatment, penetrating the venous side isn’t necessary, unlike surgery).6–8
Injection of Onyx into the feeders solidifies and blocks the arteries and veins alike leading to cure, however if high flow systems, care should be taken to ensure that complex retrograde flow from the veins into uninvolved perimedullary veins must be avoided as this can lead to catastrophic consequences.7,8
EDAVFs are rare and unusual problems which due to their rare incidence and differing presentations, are difficult to identify and treat. A thorough understanding of pathophysiology, and drainage patterns as well as a command over management options assists in optimal treatment.
None.
The author declares no conflicts of interest.

©2020 Ganapathy, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.