Journal of
eISSN: 2373-6410


Case Report Volume 9 Issue 3
1Fundación Universitaria Ciencias de la Salud- Hospital san José, Colombia
2Asistential physician of immunology laboratory Columbia clinic, Colombia
Correspondence: Carlos Andres Clavijo Prado, Fundación Universitaria Ciencias de la Salud- Hospital San José, Cra 18, Bogota, Colombia, Tel +573218174635
Received: March 24, 2017 | Published: May 7, 2019
Citation: Prado CAC, Gallego AC. Autoimmune epilepsy—a new goal of science: report of two cases in the San Jose Hospital, Bogota, Colombia. J Neurol Stroke. 2019;9(3):129-131. DOI: 10.15406/jnsk.2019.09.00362
Despite the fact that epilepsy is the third most common chronic brain disorder, relatively little is known about the processes leading to the generation of seizures. The constant advancement in technology and continuous research in epilepsy field has led the investigators to important discoveries like the immune mediated mechanisms for certain types of epilepsy. This is particularly hypothesized in patients with epilepsy of acute or subacute onset, poor responders to antiepileptic drugs, and those presenting cognitive changes or encephalopathy. A wide range of antibodies has been described in relation to the development of the disease. The following two cases were known at the San Jose Hospital in Bogota, Colombia. In this opportunity, it would be of interest to review the following cases presented at HSJ in Bogota.
Epilepsy is a health problem that affects, on average, 1% of the world population and is considered the third lead cause of neurological diseases of chronic character. The statistics do not differ from the prevalent rate in Colombia: 1.13% for the general population and 1.5% for those ages 65 and above. Epilepsy is a challenging diagnostic in the emergency department since it has multiple forms of clinical presentation.
According to literature, 20–30% of patients are refractory to medical management, and a considerable number of these patients are those whose etiology remained unknown.1
Epileptic seizures are excessive demonstrations and/or hypersynchrony of brain neurons and are usually self-limited.2
Currently, an autoimmune form of epilepsy can be diagnosed under two premises: (1) epilepsy as predominant or exclusive presentation, (2) confirmation by presence of serum antibodies or cerebrospinal fluid (CSF), inflammatory changes as a (leukocytosis or oligoclonal bands) and inflammatory changes in brain MRI (T2 hyperintense lesions and/or restriction on diffusion sequence).3–5
Although the pathophysiologic mechanism has not been fully studied or defined, patients who are not adequately controlled with the use of optimal antiepileptic drugs suggests immunogenic proceedings against neuronal antigens. These antibodies against neuronal antigens can be identified and measured, leading to diagnosis of autoimmune epilepsy. This finding provided a new vision in the differential diagnosis and generated a different therapeutic approach thus decreasing complications.6–8
Ten percent of epilepsy has autoimmune etiology. The most common is those antibodies found in potassium channels voltage-gated complexes (VGKC) comprising of 56% of the cases. LGI1 leucine-rich and glioma-inactivated 1 and contactin-associated proteinlike 2 (CASPR2). Other associated mechanisms involve antibodies to glutamic acid decarboxylase (GAD65) at 22%,9 and antibodies to brain proteins expressed receptors such as N-methyl-D-aspartate receptor (NMDAR) with a prevalence of approximately 3%. It can also be caused by antibodies against cardiolipin IgG, which can be detected using ELISA.10,11
These new scientific findings are very important since it opens a door for a new diagnosis and early therapeutic options, thus decreasing adverse outcomes. It is worthy to provide clinical tools to diagnose autoimmune epilepsy in suspected cases. Prompt diagnosis can change the management significantly.
The following two cases were presented to patients in the epilepsy unit at San Jose Hospital, Bogota, Colombia in 2016.
Twenty-nine-year-old male who was identified as a patient when was presenting memory loss, disorientation and marked drowsiness with subsequent faciobrachia dystonic seizure with a frequency of more than forty times per hour. Patient was assessed several times at a different medical centre with normal EEG and cranial MRI findings. The individual was treated as having a case of schizophrenia and did not show signs of improvement. Due to the worsening of his symptoms, patient was referred to San Jose Hospital.
Because the individual was presenting faciobrachia dystonic seizure, hence the presence of a potassium channel, mediated autoimmune epilepsy was considered. Infectious and neoplastic diseases were ruled out. Cranial MRI was normal. An EEG for twelve hours (Figure 1) was done and showed multiple episodes of faciobrachia dystonic seizure per hour accompanied by rapid path attenuation, more widespread activity during and after the episodes.
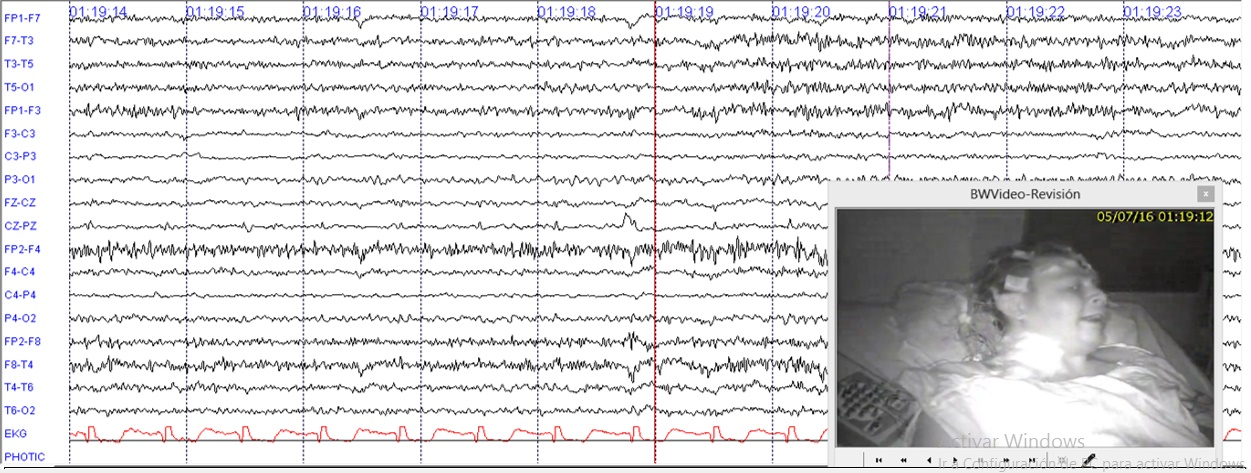
Figure 1 12 hours of EEG with LFF at 1Hz and HFF at 70Hz. Illustrates continuous fast activity mixed with muscle artefact during and after faciobrachial dystonic seizure.
The clinical diagnosis of faciobrachial dystonic seizures was confirmed by the fact that elevated voltage-gated potassium channel antibodies of 824pmol/L and positive serological test for anti-LGI1 leucine-rich and glioma-inactivated of 1 were reached. Methylprednisolone was started at a dose of 1 gram daily for five days but without improvement. The patient was given initially plasmapheresis for a term of ten sessions, displaying after that a major improvement in symptoms. He was given as well cyclophosphamide, which was shifted to rituximab due to the persistence of seizures. Seizures were treated and resolved with rituximab. Currently, he has some delay in processing information and some problems with divided and sustained visual attention.
Forty-nine-year-old female, previously healthy, was experiencing working memory loss, disorientation, inappropriate behaviour, structured visual hallucinations, and finally myoclonic seizures, which increased progressively to epilepsy encephalopathy. Infectious and neoplastic causes were ruled out. EEG for twelve hours showed continuous slowdown without adequate sleep patterns, not interictal findings (Figure 2). An immunologic cause was confirmed with a cerebral MRI showing inflammatory process described as T2 hyperintense lesions in right basal ganglia with diffusion-restricted imaging (Figure 3).
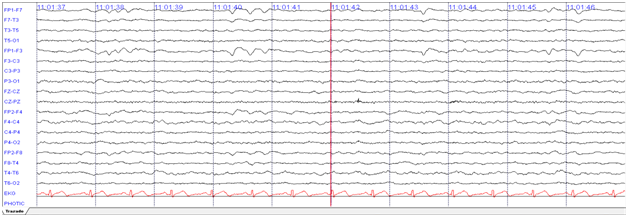
Figure 2 12 hours of EEG with LFF at 1 Hz and HFF at 70 Hz. Evidence bilateral frontal intermittent slowing complex.
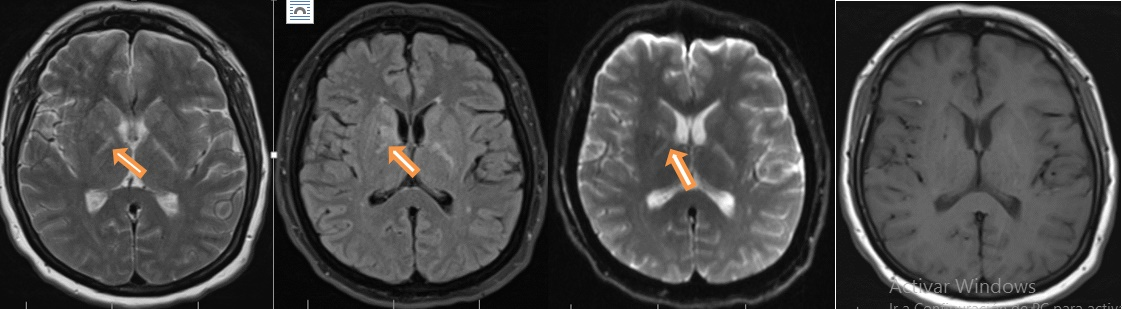
Figure 3 Hyperintense lesions on T2 and Fluid attenuated inversion recovery (FLAIR) MRI image that compromised the basal ganglia right side with restricted diffusion, no lesion enhancing.
She also had a normal CSF, but a positive serological NMDA-R antibodies test by CBA. She was initialized with pulses of methylprednisolone 1g daily for five days with slight improvement. She was then treated with plasmapheresis for five sessions and later on azathioprine 50 mg every twelve hours. Currently, she is seizure free and showed significant improvement in cognitive domains.
Autoimmune epilepsy is a condition that has been consolidated to the extent that progress is presented in the neuroimmunology as an explanation of many pathologies of the central nervous system, and it is believed that more than 10% of epileptic patients would suffer from this type of disease. Although the exact prevalence is unknown, it is more common in women, in patients with autoimmune diseases, and is associated with paraneoplastic syndromes. This large group of patients has no apparent explanation why they end up with a progressive manifestation of acute or subacute encephalopathy and become refractory to pharmacologic management. This could trigger complications such as death when proper interventions are not performed. Patients with antibodies against VGKC are usually seen in men, with a man-to-woman ratio of 2:1 and in ages over forty years old. Clinical encephalopathy has rapid onset, and 90% of patients has faciobrachial dystonic seizure. In 47% of the cases,12,13 brain MRI may be unchanged.
In cases of antibodies against NMDA-R, pathology can be documented by inflammatory changes as hyperintense lesions in T2 that restrict dissemination in neocortical region, basal ganglia, temporal mesial, or brainstem up to 33% as what happened with our patient who had positive anti NMDA-R.14,15
Electroencephalographic studies could present with three important variants: (1) interictal findings, (2) ictal findings, (3) focal slowing. But in our case, we found attenuation and generalized fast activity during and after episodes of faciobrachial seizure in VGKC patient and continuous slow complex in NMDA-R antibodies patient. There was no clear ictal or interictal findings. Most commonly, CSF can be documented with inflammatory changes by up to 31%; hyperproteinorrachia, 55%; oligoclonal bands, 19%; leucorrachia, 16%; or finally, normal 38% as in the case of our two patients.
Timely immunomodulatory therapy changes the prognosis and improves the quality of life of these patients.
In patients with acute or subacute onset of epilepsy who are resistant to standard pharmacologic management, an autoimmune etiology warrants investigation. Such that immonomodulating agents will not be delayed and fatal outcomes can be prevented.
None.
The authors of the manuscript of reference state that there is no conflict of interest related to the article.

©2019 Prado, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.