Journal of
eISSN: 2373-6410


Case Report Volume 10 Issue 5
1 Pediatric Neurologist Department of Neurosciences Fundación Cardiovascular de Colombia /Hospital Internacional de Colombia Piedecuesta, Colombia
2 General Practitioner, Department of Pediatrics, Fundación Cardiovascular de Colombia /Hospital Internacional de Colombia Piedecuesta, Colombia
Correspondence: Oscar David Peñuela Vásquez, Neurological Institute, Hospital Internacional de Colombia, Valle de Menzuly Km 7, Piedecuesta, Santander, Colombia
Received: June 16, 2020 | Published: October 30, 2020
Citation: Peñuela OD, Virachá NCB, Caicedo JC. Atypical image presentation of X-linked adrenoleukodystrophy. J Neurol Stroke. 2020;10(5):186-189. DOI: 10.15406/jnsk.2020.10.00436
Introduction: X-linked adrenoleukodystrophy (X-linked ADL-X) is the most common peroxisomal disorder affecting central nervous system, adrenal cortex and testicular functions. Central nervous system manifestations in X-linked adrenoleukodystrophy can be divided into 2 subcategories: cerebral forms that are associated with rapidly progressive inflammatory myelopathy and adrenomyeloneuropathy which is a non-inflammatory distal axonopathy. In the last quarter of the 20th century, the underlying mechanisms were described, including causal mutations of the ABCD1 gene in the disease.
Clinical case: six year old male patient with adequate neurodevelopment, history of seizure at 2 years, origin unclear. At the age of 5, he develops motor symptoms of rapid progression. First consultation for hemiparesis of the left hemicuerpus, ataxic gait, behavioral alteration. During the following year he presents symptoms of rapid progression of motor involvement; dysarthria and quadriparesis, initially asymmetric neuroimages show a progression of the lesion finally compatible with ADL-X, which is confirmed with very long chain acids and spectroscopy.
Conclusion: Radiological findings of asymmetric demyelination have rarely been described as a typical X-ALD presentation. A case with atypical radiological presentation is described to document other possibilities of radiological findings in this syndrome with metabolic involvement and to consider this type of presentation in the child population, avoiding delays in diagnosis and increased possibilities for treatment.
Keywords: X-linked adrenoleukodystrophy, spasticity, ataxia, very long chain fatty acids, demyelinating disease
The first reported cases of X-linked cerebral adrenoleukodystrophy (X-linked ADL-X) were described in the 19th century. Known as "Addison-Schilder disease" since the early 20th century, it was probably the most common brain adrenoleukodystrophy, but in the absence of biomarkers or DNA analysis evidence for that time, other diseases were often mistakenly included in this description.1,2 By 1970, Blaw introduced the term adrenoleukodystrophy as an X-linked inherited entity, and hypothesized that it was a metabolic disorder due to an enzymatic defect affecting the adrenocortical cells of the adrenal cortex and the Schwann cells of the cerebral white matter.1 One of the key lesions in patients with X-linked adrenoleukodystrophy is slowly progressive axonopathy affecting the ascending and descending motor pathways of the spinal cord, 100% penetration in men and 65% in heterozygous women at age 60.3
Adrenomyeloneuropathy is the most common variant in male patients, usually starting in their 20s and-3) 30s, and in heterozygous women starting in their 40s and 50s.2,3 Initial symptoms include progressive rigidity, decreased muscle strength and vibro-perception in the lower extremities, as well as alopecia, urinary incontinence and adrenocortical involvement (Addison's disease).4
In 60% of patients diagnosed with ADL-X, rapidly progressive inflammatory brain demyelination occurs independently of adrenomyeloneuropathy.3 The onset of inflammation is more frequent in children (35-40%), before the onset of adrenomyeloneuropathy, and less frequent (20%) in adolescents or adults, it begins most often in the midline of the corpus callosum and progresses relentlessly outward as a symmetrical, confluent lesion in both hemispheres.5
Natural male schoolboy from Venezuela, with acceptable neurodevelopment; history: product of second gestation, non-blood parents, no complications, full-term birth by cesarean section indicated by cephalopelvic disproportion, good neonatal adaptation. No relevant family history, has an older half-brother by healthy male maternal line. At the age of 2, she presents a seizure without describing semiology or recurrence of stroke events.
At 5 years of age, the strength of the left hemicorphin begins to diminish, the hemiparetic and ataxic gait is altered, and the patient is consulted in his country of origin where a simple cranial tomography scan is performed (Figure 1), which shows a hypodense parietal lesion interpreted as an ischemic stroke. Due to increased symptoms, he consulted the city of Cúcuta (Colombia) to complement his studies.
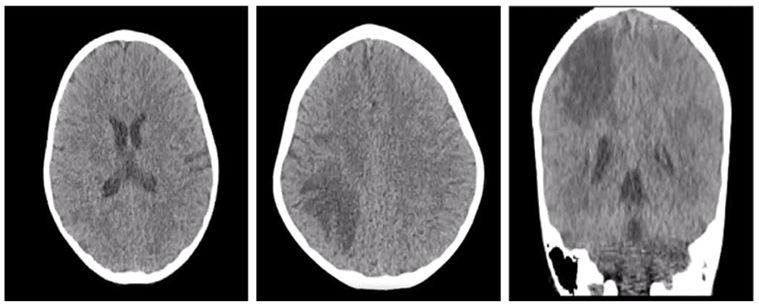
Figure 1 CT scan of the simple skull (11/July/2018) Hypodensity at the parietal region suggesting ischemic event, secondary infectious processes are not ruled out.
The first diagnosis proposed in the medical center of origin is ischemic stroke with suggestive cranial CT, corresponding studies are performed, multidisciplinary management with neurosurgery, hemato-oncology and neurology pediatric. Anticoagulant therapy with warfarin 2.5 mg every day for 3 months, rehabilitation therapies and studies to rule out hematological and autoimmune causes are indicated, with a final negative report.
The possibility of demyelinating or oncological disease is considered, oligoclonal bands and lactate/pyruvate ratio are taken with results in normal parameters. Brain biopsies reported negative for neoplasia. The patient is discharged with ambulatory evaluations.
During the following year continuous outpatient controls are proposed as a possibility of diagnosis of lysosomal deposit disease, indicating quantitative amino acids in plasma and quantitative very long chain fatty acids (VLCFA), brain resonance contrasted with spectrometry, which for reasons of health system are not performed immediately. Its clinical evolution presents deterioration to left hemiplegia that makes it impossible to walk; it also associates dysarthria. In July 2019, he was again hospitalized to reactivate studies and took a simple and contrasted brain resonance (Figure 2) in which he observed asymmetric involvement of white substance by parieto-occipital lesion with extension to thalamic region and right mesencephalic peduncle, compatible with demyelinating leukodystrophy (Hyperintense in t2 and hypointense in t1), which respects U-fibers.
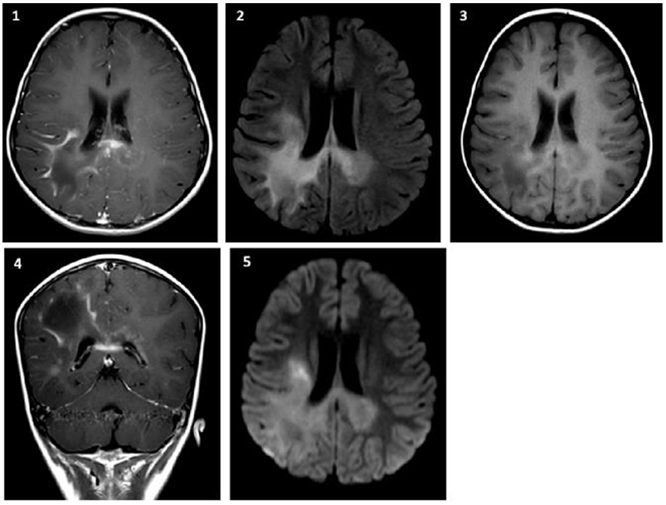
Figure 2 Simple and contrasted brain resonance (July/2019): 1. 2. FLAIR. 3. T1 simple axial. 4. Coronal T1 contrast. 5. Axial diffusion. There are areas of asymmetric bilateral and temporal parieto-occipital gliosis of greater right commitment, corpus callosum splenius, basal ganglia, midbrain and protuberance with exvacuum dilation of right ventricular atrium of probable demyelinating nature.
Admitted to the Hospital Internacional de Colombia: alert, collaborator, dysarthric, with facial asymmetry due to deviation of the labial commissure towards the right, uvula and tongue deviated towards the left, asymmetric hypertonia, hemiplegia of the left hemisphere, muscular strength 2/5 in the left hemisphere and 3/5 in the right hemisphere. Widespread exalted TMR, exhaustable right Achilles' clone and sustained left Achilles' clone. Absence of signs of meningitis. No standing, no ambulation. Hyperchromic macula in skin from retroauricular region to right periorbital region.
During hospitalization, simple and contrasted brain magnetic resonance imaging plus spectroscopy is performed (Figure 3) which confirms lysosomal disease, with increased choline and notorious alteration of the choline-creatine ratio, with associated decrease in N-acetyl aspartate indicating X-linked adrenoleukodystrophy as the main diagnosis.
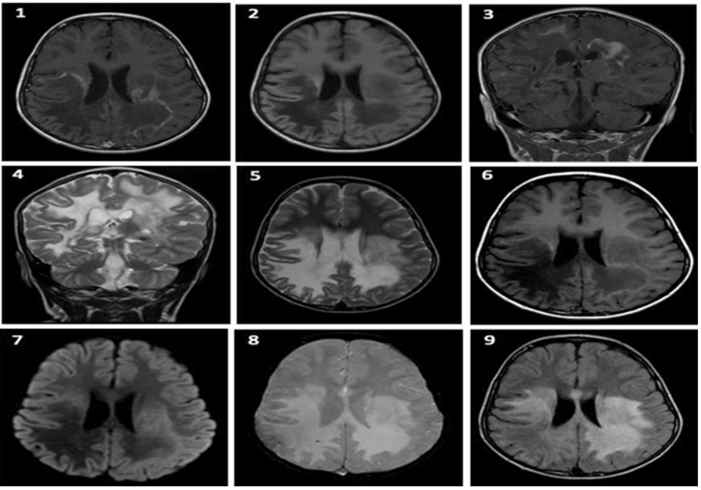
Figure 3 Simple and contrasted brain resonance with spectroscopy (31/August/2019): 1. 2. T1 simple axial. 3. Contrasted coronal T1. 4. Coronal contrast T2. 5. T2 contrasted axial. 6. T1 simple axial. 7. Diffusion axial. Axial with gradient. FLAIR axial Areas of bilateral parieto-occipital gliosis, right temporal, corpus callosum splenius, basal ganglia region, midbrain and protuberance with exvacuum dilation of right ventricular atrium of probable demyelinating nature are evidenced.
ACTH is taken with elevated report: 896.6 pg/ml (VN: 0 to 60 pg/ml), quantification of VLCFA compatible with defect in oxidation: ADL-X or adrenomyeloneuropathy (C26:0 in 1.24, C24/22 1.94 and C26/22 0.074), elevated over the normal range. Determination of the compatible ABDC1 gene, the diagnosis of ADL-X is confirmed. Referral to bone marrow transplant center to evaluate possibility of autologous transplant therapy, however, by Loes scale above the limit is discarded procedure option. Currently undergoing outpatient follow-up with pediatric neurology and endocrinology, pediatrics. His family has already received genetic and psychological counseling.
ADL-X is a genetic disease that has been described since the 19th century and is rare in our environment. It is mainly due to the mutation in the gene ABCD1 located in Xq28. Initial symptoms in X-ALD are highly variable, our patient presented acute left hemiparesis and subacute cognitive impairment. Hemiparesis, secondary to early involvement of the contralateral corticospinal tract, is not a typical manifestation at onset and may lead to a delay in diagnosis.1,3,6,7 Different forms of presentation are described, infant cerebral ADL-X, adolescent cerebral ADL-X. Adult cerebral ADL-X, adrenomyeloneuropathy (AMN).2,4,7,8
The first clinical manifestation is usually primary adrenal insufficiency.9 In terms of cerebral involvement, the typical radiological features in the infantile cerebral form of X-ALD are demyelination of the bilateral occipital white matter extending across the splenum of the corpus callosum and affecting external and anterior regions, described in a "butterfly wing" pattern on cerebral resonance, the involvement of the frontal region as a variable presentation. Approximately 80% of cases present this pattern of progression. A pattern with greater anterior involvement (asymmetric/typical) is observed in 5-20% of cases, leading to delays in diagnosis.1,10–13
Rapidly progressive inflammatory brain demyelination occurs independently of adrenomyeloneuropathy and is most common in the child population. There are several theories described in the physiopathology of this disease, however, there are still no clear data. Typically it is a slowly progressive disease, more frequent in the male population and symptoms are evident between 20 and 30 years of age, and in the female population after 40 years of age.1,3
Treatment is directed at the management of primary adrenal insufficiency, depending on the type of presentation it develops.2,14 As for brain involvement, autologous transplantation has been described as the main treatment possibility, however it is not available for all patients. It should be evaluated to define according to the type of injury, neurological involvement and staging of the disease for the time of initiation of treatment; using the LOES scale, if the score is higher than 10 it is considered that the patient will not benefit from the procedure having a higher risk of morbimortality.2,5,15,16 The use of Lorenzo's oil has shown little therapeutic utility in terms of disease progression.2
Several atypical conditions are identified in our patient: Firstly, the mode of clinical presentation, which although i t starts with motor alteration, is focal, with radiological findings in white cerebral substance, presenting an asymmetric pattern of unilateral lesion, diffuse demyelination of the white substance that in its beginning affects the right parietal lobe, with rapid progression of involvement of the cortico-spinal tract, the splenius, the corpus callosum and extending to bilateral temporo-occipital areas, predominance of right involvement and that correlates with the clinical presentation. Clearly there is no history of genetic predisposition which further deflects suspicion and early diagnosis of ADL-X.
Another variant in the clinical presentation in the patient described is the rapid progression of symptoms and rapid radiological changes, which led to rule out other more frequent pathologies of rapid progression including ischemic, autoimmune, demyelinating and neoplastic lesions. Finally, it is proposed to rule out ADL-X, a diagnosis that is made after the exclusion of other possibilities, obtaining results of VLCFA and subsequent clinical and radiological characteristics more typical that suggested greater diagnostic approach of ADL-X.
In our patient it was not possible to offer therapeutic options such as autologous transplant, due to the advanced commitment and condition of the patient by the time the diagnosis is confirmed, because it does not offer any benefit.
For all the above reasons we can assure that clinical symptoms, as well as neuroimaging findings, can be highly variable in X-ALD. We recommend determining the profile of VLCFA in all males with behavioral, motor or cognitive impairment and non-specific white matter alterations, although the presentation is not typical and infrequent in our environment, it should be considered from the beginning as a differential diagnosis. An early diagnosis of X-ALD may allow the initiation of management therapies described in the literature that have a clear impact on the prognosis of the disease or should be included in neonatal metabolic screening programs.
The authors' reason for publicizing this case study was to review the literature, document an atypical presentation of X-ALD, raise research concerns in this disease, and encourage other authors to report more case studies in the variety of their X-ALD presentations.
None.
The author declares no Conflict of interests.

©2020 Peñuela, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.