Journal of
eISSN: 2373-6410


Research Article Volume 1 Issue 2
1Institute of Neurological Sciences, Southern General Hospital, Scotland
10Medical Affairs, Life Sciences, GE Healthcare, USA
2Department of Neuroscience, University of Glasgow, Scotland
3Department of Psychiatry, University of Cambridge School of Clinical Medicine, UK
4Department of Neurology, Philipps-University of Marburg, Germany
5Department of Old Age Psychiatry, Newcastle University, UK
6Mental Health Sciences Unit, University College London, UK
7Department of Nuclear Medicine, Municipal Hospital Karlsruhe, Germany
8Department of Neurology Service, University of Barcelona, Spain
9Clinical Development, Life Sciences, GE Healthcare, USA
Correspondence: Donald G Grosset, Institute of Neurological Sciences, Southern General Hospital and Department of Neurology, University of Glasgow, Glasgow Scotland, G51 4TF, UK, Tel +44-141-201-2486, Fax +44-141-447-618
Received: May 31, 2014 | Published: June 10, 2014
Citation: Grosset DG, O’Brien JT, Oertel WH, et al. Age, gender, and diagnostic performance of ioflupane I123 injection (DaTscan™) brain imaging in patients with movement disorders and/or dementia. J Neurol Stroke. 2014;1(2):42-58. DOI: 10.15406/jnsk.2014.01.00008
Background: Although numerous studies have established the sensitivity and specificity of Ioflupane I123 Injection (ioflupane (123I), (123I) FP-CIT, DaTscan™, or DaTSCAN™) imaging in patients with movement disorders or dementia, no studies have examined the effects of gender and age on diagnostic performance.
Methods: We pooled data from four clinical trials in which patients with a movement disorder or dementia and healthy volunteers were administered ioflupane (123I). Panels of 3-5 blinded experts and/or on-site nuclear medicine physicians rated the images as normal or abnormal. Results were compared with expert clinical diagnosis (reference standard) to determine sensitivity and specificity. Subgroup and multiple logistic regression model (MLRM) analyses were performed to evaluate the effect of gender and age (two groups: <65 vs. ≥65 yrs and <75 vs. ≥75yrs) on sensitivity and specificity.
Results: There were 421 males and 305 females in the intent-to-diagnose population. Sensitivity was higher for males with parkinsonian syndrome (PS) (93.3% vs. 87.6%; P=0.0029), whereas specificity was higher for females (96.4% vs. 89.5%; P=0.0126). Sensitivity was higher in the younger age groups (<65yrs, 91.0% vs. ≥65 yrs, 86.8%; P=0.0240 and <75yrs, 90.7% vs. ≥75yrs, 78.4%, P <0.0001). Specificity was higher in subjects <65 yrs (94.0% vs. 89.7% for ≥65yrs, P=0.0384). Analysis using the 75yrs cutoff showed no differences. When MLRM was used, all covariates (disease state, age, gender, reader type, and follow-up duration) were significant predictors of the model effect on sensitivity. For specificity, only disease state and reader type were significant predictors.
Conclusions: Sensitivity and specificity of ioflupane (123I) imaging was high in all age and gender subgroups, though statistically significant differences were observed with a slightly reduced, though still diagnostically useful, sensitivity above 75yrs. This reduced sensitivity may be due to increased frequency of mixed pathologies in older people, which makes the clinical diagnosis (reference standard) less optimal. In PS, sensitivity was higher in males, whereas specificity was higher in females. MLRM analysis demonstrated that all tested covariates (including gender and age) were significant predictors for sensitivity, but not for specificity.
Keywords: Diagnostic accuracy; Sensitivity; Specificity; SPECT, Ioflupane I123 Injection; Ioflupane (123I); (123I)FP-CIT; DaTscan™; DaTSCAN™; Gender; Age; Parkinsonian syndrome; Dementia; Lewy bodies
AD, alzheimer’s disease; BIE, blinded image evaluation; CI, confidence interval; DaT, dopamine transporter; DLB, dementia with lewy bodies; ET, essential tremor; FN, false negative, FP, false positive; ITD, intent to diagnose; MSA, multiple system atrophy; NPA, negative percent agreement; PD, parkinson’s disease; PP, per protocol; PPA, positive percent agreement; PS, parkinsonian syndrome; PSP, progressive supranuclear palsy; SDD, striatal dopaminergic deficit; SDDD, striatal dopaminergic deficit disorder; SPECT, single-photon emission computed tomography; TN, true negative; TP, true positive; VaD, vascular dementia; yrs, years
The use of radiopharmaceuticals to augment clinical diagnosis of neurological diseases has facilitated greater accuracy and confidence in medical decision-making. Improved accuracy, particularly in the early stages of disease, allows for more suitable treatment selection and avoids unnecessary and potentially harmful exposure to inappropriate medications. Interpretation of the images requires expert understanding and ability to discriminate normal images from abnormal images. This expertise further relies upon the ability to understand and recognize the differences that exist and changes that occur based on gender and the normal aging process, respectively. Early studies established that changes occur normally in the brain with aging 1,2. Dopamine transporter (DaT) imaging studies performed in healthy volunteers have shown that DaT uptake decreases with age in a linear manner, declining 4.1-7.5% per decade, depending upon the region of the brain examined 3-6. Likewise, specific-to-non-specific DaT binding ratios appear to be higher in healthy females than males 4-6 and this difference is not age-related 5. In the PRamipexole On Underlying Disease (PROUD) study, a decline of 14.2%-15.5% was seen in early Parkinson’s disease over 15 months, but effects of age and gender were not analyzed 7,8.
While some knowledge exists addressing the effects of gender and age on DaT uptake in healthy volunteers, no analyses have been performed to show the effects of gender and age on diagnostic performance in patients with movement disorders or dementia. Several clinical trials have evaluated the sensitivity (equivalent to positive percent agreement (PPA)) and specificity (equivalent to negative percent agreement (NPA)) of using Ioflupane I123 Injection (ioflupane (123I) or (123I) FP-CIT or DaTSCAN™ or DaTscan™ ) and single-photon emission computed tomography (SPECT) imaging to detect the presence or absence of a striatal dopaminergic deficit (SDD) in subjects with parkinsonian syndrome (PS) and dementia with Lewy bodies (DLB) 9-15. PS and DLB may have interrelated symptoms; cognitive impairment may be present in PS and motor symptoms may manifest in subjects with DLB. Hence, evaluating the disease states individually, as well as combined, may be informative. To perform such analyses, large datasets are needed. We pooled four clinical trials presented for the US new drug application of ioflupane (123I) to examine the effects of gender and age on the diagnostic performance of ioflupane (123I) imaging.
Four clinical trials 9-12,14,15 were used for this subgroup analysis. These clinical trials had been used to support the ioflupane (123I) US new drug application. Each study had evaluated the sensitivity (PPA) and specificity (NPA) of ioflupane (123I) (Ioflupane I123 Injection or DaTscan™ or DaTSCAN™, GE Healthcare, Amersham, UK) imaging to detect the loss of dopaminergic nigrostriatal neurons in subjects thought to have a suspected movement disorder and/or dementia. The reference standard used in each of these trials was expert clinical diagnosis at baseline and 1-3 years post-scan. Table 1 summarizes the key characteristics of the four clinical trials (studies). All studies complied with the current revision of the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice Consolidated Guideline and applicable national and local laws. Ethics Committees or Institutional Review Boards approved the study protocols and amendments (Table 2). Written informed consent was given by all subjects or their guardians after the study aims, methods, anticipated benefits, and potential hazards were explained to them and prior to initiating any study procedures or assessments. The studies’ informed consents did not provide for publication of patient data; however, they included provisions for subsequent analyses, such as this subgroup analysis.
|
Study |
||||
|
Study A10 |
Study B9,11 |
Study C12 |
Study D14,15 |
|
|
Key Characteristics (Phase, Design) |
· Phase 3 · Multicenter, open-label, non-randomized · Single-dose · No control used |
· Phase 3 · Multicenter, open-label, non-randomized · Single-dose · No control used |
· Phase 3 · Multicenter, open-label, non-randomized · Repeat-dose (max. of 3) · No control used |
· Phase 4 · Multicenter, open-label, non-randomized · Single-dose · No control used |
|
Reference Standard |
· Expert clinical diagnosis at baseline according to published consensus criteria as the RCD · No clinical panel used |
· Expert clinical diagnosis at 12 months as the RCD · Clinical panel of 3 dementia experts |
· Expert clinical diagnosis at 36 months as the RCD · Review of videotapes by 2 independent movement disorder experts |
· Expert clinical diagnosis at 24 months as the RCD · No clinical panel used |
|
Subjects |
· Healthy volunteers · Subjects with Parkinson’s disease, Multiple system atrophy, Progressive supranuclear palsy, or Essential tremor |
· Subjects with dementia (possible DLB or other dementia types AD, VaD) |
· Healthy volunteers · Subjects with Early Parkinson’s disease, or Tremor (mainly essential tremor)
|
· Subjects with movement disorders (an uncertain clinical diagnosis of PS)
|
|
Objectives |
· Primary Sensitivity and specificity for detecting or excluding an SDD · Secondary Inter-reader agreement |
· Primary Sensitivity and specificity for detecting or excluding an SDD · Secondary Inter-reader agreement |
· Primary Sensitivity and specificity for detecting or excluding an SDD · Secondary Inter-reader agreement |
· Primary a Impact of ioflupane (123I) image assessments on patient diagnoses, confidence of diagnosis, and clinical management · Secondary Sensitivity and specificity for detecting or excluding an SDD |
|
Investigational product dose information |
Ioflupane (123I) 111-185 MBq (3 to 5 mCi) iv, 1 dose |
Ioflupane (123I) 111-185 MBq (3 to 5 mCi) iv, 1 dose
|
Ioflupane (123I) 111-185 MBq (3 to 5 mCi) iv, 3 doses 18 months apart |
Ioflupane (123I) 111-185 MBq (3 to 5 mCi) iv, 1 dose (73 subjects) or 2 doses 24months apart (14 subjects) |
|
No. of study sites |
6 |
40 |
10 |
15 |
|
No. of subjects entered in the study/completed efficacy evaluations |
250/220 |
351/288 |
202/102 |
125/118 |
|
Age of ITD population, range (mean) |
40, 80 (62.7) |
54, 90 (73.9)
|
33, 79 (60.4) |
25, 84 (64.2) |
|
Gender |
62% male, 38% female |
57% male, 43% female
|
56% male, 44% female |
53% male, 47% female |
|
Blinded image evaluations performed |
Yes (5 central readers) |
Yes (3 central readers) |
Yes (3 central readers) |
No (on-site readers only) |
Table 1 Key characteristics of four clinical studies included in age and gender reported analysis
aPrimary objective was to assess clinical utility of ioflupane (123I) images, however, images were used for age and gender subgroup analysis. AD, alzheimer’s disease; DLB, dementia with lewy bodies; ITD, intent to diagnose; MBq, megabecquerel; PS, parkinsonian syndrome; RCD, reference clinical diagnosis; SDD, striatal dominergic deficit; VaD, vascular dementia.
|
Study A |
|||
|
Committee Name |
City |
Country |
Chairman |
|
Medical Research Ethics Committee, The Phillips University Clinic |
Marburg |
Germany |
Dr. P Heubel |
|
The Faculty of Medicine Ethics Committee, Ludwig Maximilian University of Munich |
Munich |
Germany |
Prof. Dr. med. Dent. W Gernet |
|
Southern General Hospital Medical Ethics Committee |
Glasgow |
UK |
Rev. D Keddie |
|
Medical Ethics Committee, Academic Medical Center, Amsterdam University |
Amsterdam |
The Netherlands |
Prof. L Arisz |
|
Joint UCL/UCLH Committees on the Ethics of Human Research |
London |
UK |
Prof. A McLean |
|
Ethics Review Committee, University Hospital |
Ghent |
Belgium |
Prof. Dr. M Bogaert |
|
Ethikkommission des Landes Oberösterreich |
Linz |
Austria |
Univ. Prof. Prim Dr. Fisher |
|
Ethik-Kommission der Medizinischen Fakultät der Universität Wien und des Allgemeinen Krnkenhauses der Stadt Wien AKH |
Wien |
Austria |
Univ. Prof. Dr. E Singer |
|
Comité consultative pour la protection des personnes dans la recherché biomédicale Bordeaux B |
Bordeaux |
France |
Prof. MC Saux |
|
Ethik-Kommission an der Medizinischen Fakultät der Universität Leipzig |
Leipzig |
Germany |
Prof. Dr. med. R Preiß |
|
Ethikkommission, Campus Charité Mitte |
Berlin |
Germany |
Prof. Dr. med. R Uebelhack |
|
Ethik-Kommission der Ruhr- Universität Bochum, Medizinischen Fakultät |
Bochum |
Germany |
Prof. Dr. Zenz |
|
Ethik-Kommission der Georg-August-Ruhr-Universität Göttingen |
Göttingen |
Germany |
Prof. Dr. med. E Rüther |
|
Ethik-Kommission der Ärztekammer Hamburg |
Hamburg |
Germany |
Prof. Dr. med. Th. Weber |
|
Medizinischen Hochschule Hannover, Ethikkommission |
Hannover |
Germany |
Prof. Dr. HD Tröger |
|
Landesärztekammer Rheinland-Pfalz, Ethikkommission |
Mainz |
Germany |
Prof. Dr. Rittner |
|
Kommission für Ethik in der ärztlichen Forschung. Bereich Humanmedizin, Klinikum der Philipps- Universität Marburg |
Marburg |
Germany |
Prof. Dr. Med. G Richter |
|
Regione Veneto, Aziendo Ospedaliera di Padova, Comitato Etico per la Sperimentazione |
Padova |
Italy |
Dr. R Pegoraro |
|
Azienda Ospedaliera Pisana, Comitato etico per la studio del farmaco sull’ uomo |
Pisa |
Italy |
Prof. R Barsotti |
|
Regional komité for medisinsk forskninsetikk, Vest-Norge (REK Vest), Universitetet i Bergen, det medisinske fakultet |
Bergen |
Norway |
A Berstad |
|
Comité Ético de Investigaçáo Clinica |
Porto |
Portugal |
|
|
Karolinska Institutet, Forskningsettikkommitté Syd |
Stockholm |
Sweden |
Prof. H Glaumann |
|
Regionala etikprövningsnämnden i Stockholm |
Stockholm |
Sweden |
Prof. LE Rutquist |
|
Clinic Barcelona, Hospital Universitari, Comitè ètic investigaciò clinica |
Barcelona |
Spain |
|
|
Comité Etico de Investigación Clinica, Hospital Universitario de Getafe |
Madrid |
Spain |
|
|
Comité etico de investigación clinica Hospital “La Fe” Valencia |
Valencia |
Spain |
|
|
Northern and Yorkshire Multi-Centre Ethics Committee, Durham University |
Durham |
UK |
J Kelly/S Brunton-Shiels |
|
Gateshead Local research Ethics Committee |
Sunderland |
UK |
Dr. DG Raw |
|
Northumberland, Tyne and Wear NHS Strategic Health Authority Local Research Ethics Committees, Newcastle General Hospital |
Newcastle upon Tyne |
UK |
Dr. J Lothian, PD Carr |
|
Southampton & South West Hampshire Local Research Ethics Committee |
Southampton |
UK |
C Wright |
|
Ethikkommission der Medizinischen Fakultät der Ludwig-Maximilans-Universität, LMU, Klinikum Großhadern |
München |
Germany |
Prof. Dr. G Paumgartner |
|
Ethikkommission der Fakultät für Medizin der Technischen Universität München |
München |
Germany |
Prof. Dr. A Schömig |
|
Aligemeines öffentliches Krankenhaus der Stadt Linz, Kommission zur Beurteilung klinischer Prüfungen von Arzneimitteln, Ethikkommission |
Linz |
Austria |
Primar Dr. H Stekel |
|
Ospedali Civili Brescia, Aziendo Ospedaliera, Comitato Etico |
Brescia |
Italy |
Prof. F De Ferrari |
|
Fakultní nemocnice v Motole, Etickákomise |
Prague |
Czech Republic |
MUDr. V Šmelhaus |
|
Brighton and Sussex Local Research Ethics Committee |
Brighton |
UK |
Dr. P Seddon |
|
East Sussex Local Research Ethics Committee |
Brighton |
UK |
Dr. J Rademaker |
|
South Manchester Local Research Ethics Committee |
Manchester |
UK |
Dr. W Pettit |
|
Central Manchester Research Ethics Committee |
Manchester |
UK |
Dr. D Mandal |
|
NHS Tayside Board, Tayside Committee on Medical Research Ethics, Ninewells Hospital & Medical School |
Dundee |
UK |
NF Brown |
|
Fazio-Fondazione San Raffaele Del Monte Tabor Milano, Comitato Etico Dell’istituto Nazionale Neurologico Besta di Milano |
Milano |
Italy |
Prof. E Müller |
|
IRCCS – Fondazione San Raffaele Del Monte Tabor di Milano |
Milano |
Italy |
Prof. G Zoppei |
|
Comité ético de investigación clínica, Servicio Andaluz de Salud, Consejería de Salud, Hospitales Universitarios Virgen de Rocío de Sevilla |
Sevilla |
Spain |
|
|
Ethikkommission der stadt Wien |
Wien |
Austria |
Dr. H Serban |
|
North Sheffield Local Research Ethics Committee, Northern General Hospital |
Sheffield |
UK |
Dr. GPM Clark |
|
Glasgow West Local Research Ethics Committee |
Glasgow |
UK |
Dr. J Hunter |
|
NHS Greater Glasgow Primary Care Division Local Research Ethics Committee, Gartnavel Royal Hospital |
Glasgow |
UK |
Dr. P Fleming |
|
Frenchay Research Ethics Committee, North Bristol NHS Trust Headquarters |
Bristol |
UK |
Drs. J Kendall and M Shere |
|
Ärztekammer Berlin, Ethik-Kommission |
Berlin |
Germany |
C Biondo |
|
Ethikkommission des Landes Bremen, Institut für Klinische Pharmaakologie, Klinikum Bremen-Mitte |
Bremen |
Germany |
Dr. K Boomgaarden-Brandes |
|
Ethikkommission der Fakultät für Medizin der Technischen Universität München |
München |
Germany |
Prof. Dr. A Schömig |
|
Ethics Committee of the Southern General Hospital NHS Trust, Glasgow |
Glasgow |
UK |
Rev. D Keddie |
|
Kommission für Ethik in der Ärztlichen Forschung, Klinikum der Philipps-Universität Marburg |
Marburg |
Germany |
Prof. Dr. med. G Richter |
|
New Cross Hospital Local Research Ethics Committee |
Wolverhampton |
UK |
DJ Little |
|
Southampton and South West Hampshire Joint Local |
Southampton |
UK |
Dr. A Kermode |
|
Joint Ethics Committee Newcastle and North Tyneside Health Authority |
Newcastle |
UK |
Prof. PA Heasman |
|
Comite Etico de Investigacion Clinica Hospital Clinic I Provincial |
Barcelona |
Spain |
Prof. J Rodes |
|
Comite Etico de Investigacion Clinica del Hospital de la Santa Creu I Sant Pau |
Barcelona |
Spain |
FJ Carrenca |
|
Comité d’ éthique hospitalier, Cliniques Universitaires de Mont-Godinne |
Yvoir |
Belgium |
Dr. P Evrard |
|
Hospitais da Universidade de Coimbra |
Coimbra |
Portugal |
Dr. JA Branquinho de Carvalho |
|
Ethikkommission der Medizinischen Faultät der Universität Innsbruck |
Innsbruck |
Austria |
Univ. Prof. Dr. P Lukas |
|
Hospital Ethical Committee, University Hospital UCL Mont-Godinne |
Yvoir |
Belgium |
Dr. P Evrard |
|
Commission for Ethics, AZ St.-Jan AV |
Brugge |
Belgium |
Dr. J Van Droogenbroeck |
|
Comite Consultatif de Protection des Personnes Dans La Recherche Biomedicale de Lille, Hôpital Huriez |
Lille |
France |
Prof. PY Hatron |
|
Ethik-Kommission der Ärztekammer Hamburg Kōrperschaft des ōffentlichen Rechts |
Hamburg |
Germany |
Prof. Dr. Med. K Held |
|
Ethikkomission des Klinikums der Universität Regensberg |
Regensberg |
Germany |
Prof. Dr. R Andreesen |
|
Vorsitzenden der Ethikkommission Bei der Ärztekammer des Saarlandes |
Saarbrücken |
Germany |
Dr. S Ertz |
|
Spett. Le Comitato Etico |
Milano |
Italy |
Prof. A Randazzo |
|
Comitato Etico Per La Sperimentazione Clinica Del Farmaci |
Firenze |
Italy |
Prof. L Zilletti |
|
Ministério Da Saúde Hospitais Da Universidade De Coimbra |
Coimbra |
Portugal |
Prof. Dr. JM Pedroso Lima |
|
Comité Ético De Investigación Clínica Hospital Clínic I Provincial |
Barcelona |
Spain |
Prof. MA Asenjo Sebastián |
|
Comité Ético De Investigación Clínica Del Hospital De La Santa Creu I Sant Pau |
Barcelona |
Spain |
FJ Carrencá |
|
King’s College Hospital |
London |
UK |
Prof. ER Howard |
|
Southampton and South West Hampshire Local Research Ethics Committees |
Southampton |
UK |
Dr. A Kermode |
|
Etik-Kommission Der Medizinischen Faultät der Universität Wien |
Wien |
Austria |
Univ. Prof. Dr. E Singer |
Table 2 Ethics committees for the four studies in the sub group analysis.
Procedures
Each of the four studies key results have been published 9-12,14,15, but not age and gender effects. Briefly, they were open-label, non-randomized, Phase 3 or 4 clinical trials to determine the sensitivity and specificity of ioflupane (123I) imaging to detect or exclude an SDD in subjects with a movement disorder (PS, including Parkinson’s Disease (PD), multiple system atrophy (MSA) and progressive supranuclear palsy (PSP); or essential tremor (ET) and/or dementia (DLB, Alzheimer’s disease (AD) or vascular dementia (VaD); and healthy volunteers. Subjects received either one (Studies A and B) or up to 3 (Study C and D [no more than 2) doses of 111-185 MBq of ioflupane (123I). SPECT imaging was performed 3-6 hours after injection. Ioflupane (123I) images were read on-site (institutional reads) in all 4 studies. In 3 of the studies, images were also read by 3 or 5 independent blinded readers (blinded image evaluation, BIE). Images were classified as normal (SDD absent) or abnormal (SDD present). Expert clinical diagnosis made by neurologists or dementia specialists blinded to imaging results established whether the subject had an SDDD (PD, PS, PSP, MSA, or DLB) or did not have an SDDD (ET, AD or VaD and healthy volunteers). Ioflupane (123I) image results were compared with the corresponding subject’s reference clinical diagnosis, made at baseline and 1-3 years post-scan, and classified as True Positive (TP), True Negative (TN), False Positive (FP) or False Negative (FN).
Statistical analysis
All statistical analyses were performed using Statistical Analysis Software (SAS Institute Inc., Cary, NC, USA). Demographic data are presented using descriptive statistics. Populations evaluated in this subgroup analysis were the Intent to diagnose (ITD; all dosed subjects who underwent SPECT imaging and underwent the reference clinical diagnosis assessment for the relevant analysis) and Per protocol (PP; all subjects in the ITD population with no major protocol violations). Sensitivity was calculated as nTP / (nTP+nFN), (n = number of subjects). Specificity was calculated as nTN / (nTN+nFP). Sensitivity (PPA) and specificity (NPA) were calculated for the ITD and PP populations, and are reported with 95% confidence intervals (CI). Subgroup analyses were performed based on gender (male/female) and age, using 2 arbitrarily set thresholds,
Study participants
The ITD population comprised 726 subjects and the PP population, 622. Subject baseline demographic characteristics and reference clinical diagnosis are shown by study and for the total ITD population in Table 3 and PP population in Table 4. There were no meaningful differences in baseline characteristics between the ITD and PP populations.
|
Study |
||||||
|
|
|
Study A (N = 220) |
Study B (N = 326) |
Study C (N = 102) |
Study D (N=78) |
Total (N = 726) |
|
Age (yr) |
Mean (SD) Min, Max Median |
62.7 (8.87) 40, 80 63.5 |
73.9 (7.17) 54, 90 75.0 |
60.4 (10.91) 33, 79 61.0 |
64.2 (11.99) 25, 84 67.0 |
67.6 (10.60) 25, 90 69.0 |
|
Gender |
Male Female |
136 (62%) 84 (38%) |
187 (57%) 139 (43%) |
57 (56%) 45 (44%) |
41 (53%) 37 (47%) |
421 (58%) 305 (42%) |
|
Race |
Caucasian Black Asian Other |
216 (98%) 3 (1%) 1 (<1%) 0 (0%) |
326 (100%) 0 (0%) 0 (0%) 0 (0%) |
102 (100%) 0 (0%) 0 (0%) 0 (0%) |
77 (99%) 0 (0%) 1 (1%) 0 (0%) |
721 (99%) 3 (<1%) 2 (<1%) 0 (0%) |
|
PS (SDDD) Possible PS Probable PS |
158 (72%) 158 (72%) 0 (0%) |
0 (0%) 0 (0%) 0 (0%) |
71 (70%) 5 (5%) 66 (65%) |
48 (62%) 48 (62%) 0 (0%) |
277 (38%) 211 (29%) 66 (9%) |
|
|
DLB (SDDD) Possible DLB Probable DLB |
0 (0%) 0 (0%) 0 (0%) |
116 (36%) 27 (8%) 89 (27%) |
0 (0%) 0 (0%) 0 (0%) |
0 (0%) 0 (0%) 0 (0%) |
116 (16%) 27 (4%) 89 (12%) |
|
|
Non-PS/Non-DLB (no SDD) ET AD Other |
62 (28%) 27 (12%) 0 (0%) 35 (16%) |
126 (39%) 0 (0%) 125 (38%) 1 (<1%) |
31 (30%) 14 (14%) 0 (0%) 17 (17%) |
30 (38%) 23 (29%) 0 (0%) 7 (9%) |
249 (34%) 64 (9%) 125 (17%) 60 (8%) |
|
|
SDD Presenta SDD Absent |
158 (72%) 62 (28%) |
116 (36%)b 126 (39%)b |
71 (70%) 31 (30%) |
48 (62%) 30 (38%) |
393 (54%) 249 (34%) |
|
Table 3 Subject baseline demographics and clinical diagnosis (Reference Clinical Diagnosis) by study – ITD population
aIncludes Possible and Probable PS and Possible and Probable DLB diagnoses.
b22 subjects had no diagnosis, and 62 subjects were not assessed at 12-month visit.
AD, alzheimer’s disease; DLB, dementia with Lewy bodies; ET, essential tremor; ITD, intent to diagnose; N, number of subjects in the study; PS, parkinsonian syndrome; SD, standard deviation; SDD, striatal dopaminergic deficit; SDDD, striatal dopaminergic deficit disorder; yr, year.
|
Study |
||||||
|
|
|
Study A (N = 157) |
Study B (N = 288) |
Study C (N = 100) |
Study D (N=77) |
Total (N = 622) |
|
Age (yr) |
Mean (SD) Min, Max Median |
63.1 (8.51) 40, 80 64.0 |
74.2 (7.02) 54, 90 75.0 |
60.5 (10.97) 33, 79 61.5 |
64.1 (12.05) 25, 84 67.0 |
67.9 (10.61) 25, 90 69.0 |
|
Gender |
Male Female |
99 (63%) 58 (37%) |
160 (56%) 128 (44%) |
57 (57%) 43 (43%) |
40 (52%) 37 (48%) |
356 (57%) 266 (43%) |
|
Race |
Caucasian Black Asian Other |
153 (97%) 3 (2%) 1 (1%) 0 (0%) |
288 (100%) 0 (0%) 0 (0%) 0 (0%) |
100 (100%) 0 (0%) 0 (0%) 0 (0%) |
76 (99%) 0 (0%) 1 (1%) 0 (0%) |
617 (99%) 3 (<1%) 2 (<1%) 0 (0%) |
|
PS (SDDD) Possible PS Probable PS |
115 (73%) 115 (73%) 0 (0%) |
0 (0%) 0 (0%) 0 (0%) |
69 (69%) 5 (5%) 64 (64%) |
47 (61%) 47 (61%) 0 (0%) |
231 (37%) 167 (27%) 64 (10%) |
|
|
DLB (SDDD) Possible DLB Probable DLB |
0 (0%) 0 (0%) 0 (0%) |
110 (38%) 25 (9%) 85 (30%) |
0 (0%) 0 (0%) 0 (0%) |
0 (0%) 0 (0%) 0 (0%) |
110 (18%) 25 (4%) 85 (14%) |
|
|
Non-PS/Non-DLB (no SDDD) ET AD Other |
42 (27%) 16 (10%) 0 (0%) 26 (17%) |
123 (43%) 0 (0%) 122 (42%) 1 (<1%) |
31 (31%) 14 (14%) 0 (0%) 17 (17%) |
30 (39%) 23 (30%) 0 (0%) 7 (9%) |
226 (36%) 53 (9%) 122 (20%) 51 (8%) |
|
|
SDDD Presenta SDDD Absent |
115 (73%) 42 (27%) |
110 (38%) 123 (43%) |
69 (69%) 31 (31%) |
47 (61%) 30 (39%) |
341 (55%) 226 (36%) |
|
Table 4 Demographic characteristics and clinical diagnosis (per Reference Clinical Diagnosis) by study – PP population
aIncludes Possible and Probable PS and Possible and Probable DLB diagnoses.
AD, alzheimer’s disease; DLB, dementia with lewy bodies; ET, essential tremor; n = number of subjects in the study; PP, per protocol; PS, parkinsonian syndrome; SD, standard deviation; SDDD, striatal dopaminergic deficit disorder.
Effect of gender on diagnostic performance
Figure 1 displays sensitivity (PPA) and specificity (NPA) in males vs. females in subjects with PS and DLB (ITD population), measured at baseline and upon follow-up, using BIE results. There were statistically significant differences at baseline in subjects with PS, with sensitivity being lower and specificity higher in females compared with males. This was also true for the whole population (Table 5). At month 18 and month 36, statistically significant differences were not observed. There were no differences between males and females observed in subjects with DLB at any time points. When on-site image reads were used (Table 6), no statistically significant differences were observed between males and females.
|
Reference Clinical Diagnosis |
||||||
|
|
Parkinsonian Syndrome (PS; SDDD) |
Dementia with Lewy Bodies (DLB; SDDD) |
Whole Population |
|||
|
|
Sensitivity % (95% CI) |
Specificity % (95% CI) |
Sensitivity % (95% CI) |
Specificity % (95% CI) |
Sensitivity % (95% CI) |
Specificity % (95% CI) |
|
Baselinea Male (n=318)
Female (n=235)
P value |
93.3 (91.0, 95.1) 87.6 (83.9, 90.7) 0.0029 |
89.5 (84.9, 93.1) 96.4 (92.3, 98.7) 0.0126 |
81.0 (73.7, 87.0) 74.4 (64.2, 83.1) 0.2568 |
87.6 (82.3, 91.8) 92.4 (88.0, 95.6) 0.1378 |
90.9% (88.6, 92.8) 85.1 (81.6, 88.2) 0.0023 |
88.6 (85.3, 91.4) 94.1 (91.3, 96.3) 0.0062 |
|
Month 12b Male (n=115)
Female (n=94)
P value |
|
|
79.7 (72.3, 85.9) 76.4 (66.2, 84.8) 0.6248 |
92.7 (87.8, 96.0) 92.9 (88.1, 96.1) 1.0000 |
|
|
|
Month 18c Male (n=57)
Female (n=45)
P value |
78.7 (69.8, 86.0) 79.0 (70.0, 86.4) 1.0000 |
93.7 (84.5, 98.2) 100 (88.1, 100) 0.3041 |
|
|
|
|
|
Month 36c Male (n=55)
Female (n=44)
P value |
75.7 (66.3, 83.6) 77.6 (68.0, 85.4) 0.8678 |
95.0 (86.1, 99.0) 100 (88.4, 100) 0.5480 |
|
|
|
|
Table 5 Sensitivity (PPA) and specificity (NPA) from blinded image evaluation between male and female subjects. Values are mean results across all readers. Intent to diagnose population
aSummary result calculated across all readers for Studies A, B, and C at baseline.
bSummary result calculated across all readers for Study B.
cSummary result calculated across all readers for Study C.
Sensitivity (PPA)/specificity (NPA) for parkinsonian syndrome (PS) was calculated based on PS present vs. PS absent; Sensitivity (PPA)/specificity (NPA) for dementia with Lewy bodies (DLB) was calculated based on Probably DLB vs. Non-DLB; Sensitivity (PPA)/specificity (NPA) for whole population was calculated based on SDD present vs. SDD absent.
P value is from Fisher’s exact test.
CI, confidence interval; n, number of subjects included in the analysis; NPA, negative percent agreement; PPA, positive percent agreement; SDDD, striatal dopaminergic deficit disorder
|
Reference Clinical Diagnosis |
||||||
|
|
Parkinsonian Syndrome (PS; SDDD) |
Dementia with Lewy Bodies (DLB; SDDD) |
Whole Population |
|||
|
|
Sensitivity % (95% CI) |
Specificity % (95% CI) |
Sensitivity % (95% CI) |
Specificity % (95% CI) |
Sensitivity % (95% CI) |
Specificity % (95% CI) |
|
Mean Resultsa Male (n=352)
Female (n=262)
P value |
91.3 (86.8, 94.6) 87.6 (82.0, 92.0) 0.2583 |
93.0 (86.6, 96.9) 85.5 (75.0, 92.8) 0.1255 |
90.9 (80.0, 97.0) 88.2 (72.5, 96.7) 0.7271 |
82.5 (70.9, 90.9) 80.6 (68.6, 89.6) 0.8209 |
91.2 (87.3, 94.2) 87.7 (82.6, 91.8) 0.2379 |
89.3 (83.7, 93.4) 83.2 (75.7, 89.2) 0.1300 |
|
Month 12b Male (n=118)
Female (n=96)
P value |
|
|
90.9 (80.0, 97.0) 88.2 (72.5, 96.7) 0.7271 |
82.5 (70.9, 90.9) 80.6 (68.6, 89.6) 0.8209 |
|
|
|
Month 18c Male (n=56)
Female (n=45)
P value |
82.9 (66.4, 93.4) 80.0 (63.1, 91.6) 1.0000 |
95.2 (76.2, 99.9) 80.0 (44.4, 97.5) 0.2369 |
|
|
|
|
|
Month 36c Male (n=53)
Female (n=44)
P value |
82.4 (65.5, 93.2) 85.3 (68.9, 95.0) 1.0000 |
89.5 (66.9, 98.7) 80.0 (44.4, 97.5) 0.5920 |
|
|
|
|
Table 6 Sensitivity (PPA) and specificity (NPA) from on-site image reads between male and female subjects Intent to diagnose population.
aSummary results calculated across all studies and time points. For Study B, month 12 reference clinical diagnosis was used.
bSummary result calculated for Study B.
cSummary result calculated for Study C.
Sensitivity (PPA)/specificity (NPA) for parkinsonian syndrome (PS) was calculated based on PS present vs. PS absent; Sensitivity (PPA)/specificity (NPA) for dementia with Lewy bodies (DLB) was calculated based on Probably DLB vs. Non-DLB; Sensitivity (PPA)/specificity (NPA) for whole population was calculated based on SDD present vs. SDD absent.
P value is from Fisher’s exact test.
CI, confidence interval; n, number of subjects included in the analysis; NPA, negative percent agreement; PPA, positive percent agreement; SDDD, striatal dopaminergic deficit disorder
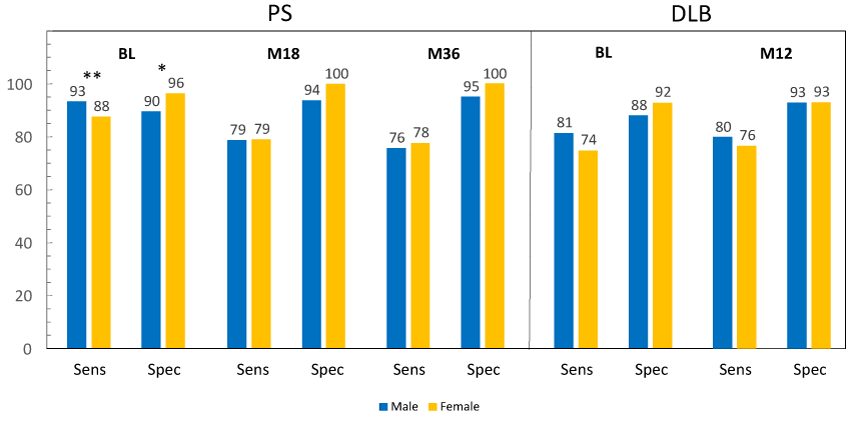
Figure 1 Sensitivity (PPA) and specificity (NPA) in males vs. females with PS and DLB.
Sensitivity (PPA) and specificity (NPA), read at baseline (BL), and at month 18 (M18) and month 36 (M36) in subjects with PS (parkinsonian syndrome) and at month 12 (M12) in subjects with DLB (dementia with Lewy bodies). *P <0.05; **P <0.01.
Effect of age on diagnostic performance
Figure 2 displays sensitivity (PPA) and specificity (NPA) in subjects < and ≥ 65years (yrs) and 75yrs, broken out by disease state (PS and DLB), in which BIE reads at baseline were used. In subjects with PS, sensitivity and specificity was lower in older subjects than younger subjects, however the difference was only statistically significant for specificity when using 65yrs as the cutoff. In subjects with DLB, sensitivity was higher in older subjects when using 65 yrs as the cutoff, but lower if 75yrs was used. Sensitivity remained statistically significantly higher in older subjects using the 65-yr cutoff at the Month 12 assessment. Specificity was roughly equivalent, regardless of which age cutoff was used. In the whole population, sensitivity was statistically significantly higher in younger subjects using either cutoff; specificity was only higher in younger subjects when 65 yrs was used as the cutoff (Tables 7&8). When the on-site image reads were used, the only statistically significant differences noted were that specificity was higher at baseline in younger subjects using the 65-yr cutoff and the whole population (Tables 9 and 10).
|
Reference Clinical Diagnosis |
||||||
|
|
Parkinsonian Syndrome (PS; SDDD) |
Dementia with Lewy Bodies (DLB; SDDD) |
Whole Population |
|||
|
|
Sensitivity % (95% CI) |
Specificity % (95% CI) |
Sensitivity % (95% CI) |
Specificity % (95% CI) |
Sensitivity % (95% CI) |
Specificity % (95% CI) |
|
Baselinea <65 yrs (n=204)
65 yrs (n=349)
P value |
92.3 (89.7, 94.4) 89.7 (86.5, 92.3) 0.1808 |
94.8 (91.1, 97.3) 89.0 (83.4, 93.3) 0.0378 |
57.1 (34.0, 78.2) 80.6 (74.6, 85.6) 0.0226 |
90.7 (79.7, 96.9) 90.0 (86.4, 92.9) 1.0000 |
91.0 (88.3, 93.2) 86.8 (84.0, 89.2) 0.0240 |
94.0 (90.6, 96.5) 89.7 (86.8, 92.1) 0.0384 |
|
Month 12b <65 yrs (n=23)
³65 yrs (n=186)
P value |
|
|
47.6 (25.7, 70.2) 81.5 (75.6, 86.4) 0.0011 |
89.6 (77.3, 96.5) 93.2 (89.9, 95.8) 0.3682 |
|
|
|
Month 18c <65 yrs (n=60)
65 yrs (n=42)
P value |
83.3 (75.4, 89.5) 73.1 (62.9, 81.8) 0.0903 |
94.9 (85.9, 98.9) 97.0 (84.2, 99.9) 1.0000 |
|
|
|
|
|
Month 36c <65 yrs (n=58)
³65 yrs (n=41)
P value |
80.7 (72.3, 87.5) 71.3, (60.6, 80.5) 0.1320 |
94.7 (85.4, 98.9) 100 (89.4, 100) 0.2955 |
|
|
|
|
Table 7 Sensitivity (PPA) and specificity (NPA) from blinded image evaluation between < 65 and ≥ 65 yrs subjects Values are mean results across all readers. Intent to diagnose population
aSummary result calculated across all readers for Studies A, B, and C at baseline.
bSummary result calculated across all readers for Study B.
cSummary result calculated across all readers for Study C.
Sensitivity (PPA)/specificity (NPA) for parkinsonian syndrome (PS) was calculated based on PS present vs. PS absent; Sensitivity (PPA)/specificity (NPA) for dementia with Lewy bodies (DLB) was calculated based on Probably DLB vs. Non-DLB; Sensitivity (PPA)/specificity (NPA) for whole population was calculated based on SDD present vs. SDD absent.
P value is from Fisher’s exact test.
CI, confidence interval; n, number of subjects included in the analysis; NPA, negative percent agreement; PPA, positive percent agreement; SDDD, striatal dopaminergic deficit disorder.
|
Reference Clinical Diagnosis |
||||||
|
|
Parkinsonian Syndrome (PS; SDDD) |
Dementia with Lewy Bodies (DLB; SDDD) |
Whole Population |
|||
|
|
Sensitivity % (95% CI) |
Specificity % (95% CI) |
Sensitivity % (95% CI) |
Specificity % (95% CI) |
Sensitivity % (95% CI) |
Specificity % (95% CI) |
|
Baselinea <65 yrs (n=204)
³65 yrs (n=349)
P value |
92.3 (89.7, 94.4) 89.7 (86.5, 92.3) 0.1808 |
94.8 (91.1, 97.3) 89.0 (83.4, 93.3) 0.0378 |
57.1 (34.0, 78.2) 80.6 (74.6, 85.6) 0.0226 |
90.7 (79.7, 96.9) 90.0 (86.4, 92.9) 1.0000 |
91.0 (88.3, 93.2) 86.8 (84.0, 89.2) 0.0240 |
94.0 (90.6, 96.5) 89.7 (86.8, 92.1) 0.0384 |
|
Month 12b <65 yrs (n=23)
³65 yrs (n=186)
P value |
|
|
47.6 (25.7, 70.2) 81.5 (75.6, 86.4) 0.0011 |
89.6 (77.3, 96.5) 93.2 (89.9, 95.8) 0.3682 |
|
|
|
Month 18c <65 yrs (n=60)
³65 yrs (n=42)
P value |
83.3 (75.4, 89.5) 73.1 (62.9, 81.8) 0.0903 |
94.9 (85.9, 98.9) 97.0 (84.2, 99.9) 1.0000 |
|
|
|
|
|
Month 36c <65 yrs (n=58)
³65 yrs (n=41)
P value |
80.7 (72.3, 87.5) 71.3, (60.6, 80.5) 0.1320 |
94.7 (85.4, 98.9) 100 (89.4, 100) 0.2955 |
|
|
|
|
Table 8 Sensitivity (PPA) and specificity (NPA) from blinded image evaluation between < 75 and ≥ 75yrs subjects. Values are mean results across all readers. Intent to diagnose population
aSummary result calculated across all readers for Studies A, B, and C at baseline.
bSummary result calculated across all readers for Study B.
cSummary result calculated across all readers for Study C.
Sensitivity (PPA)/specificity (NPA) for parkinsonian syndrome (PS) was calculated based on PS present vs. PS absent; Sensitivity (PPA)/specificity (NPA) for dementia with Lewy bodies (DLB) was calculated based on Probably DLB vs. Non-DLB; Sensitivity (PPA)/specificity (NPA) for whole population was calculated based on SDD present vs. SDD absent.
P value is from Fisher’s exact test.
CI, confidence interval; n, number of subjects included in the analysis; NPA, negative percent agreement; PPA, positive percent agreement; SDDD, striatal dopaminergic deficit disorder.
|
Reference Clinical Diagnosis |
||||||
|
|
Parkinsonian Syndrome (PS; SDDD) |
Dementia with Lewy Bodies (DLB; SDDD) |
Whole Population |
|||
|
|
Sensitivity % (95% CI) |
Specificity % (95% CI) |
Sensitivity % (95% CI) |
Specificity % (95% CI) |
Sensitivity % (95% CI) |
Specificity % (95% CI) |
|
Mean Resultsa <65 yrs (n=235)
³65 yrs (n=379)
P value |
89.7 (85.0, 93.4) 89.5 (84.3, 93.5) 1.0000 |
94.2 (87.8, 97.8) 85.0 (75.3, 92.0) 0.0468 |
77.8 (40.0, 97.2) 91.3 (82.8, 96.4) 0.2247 |
81.3 (54.4, 96.0) 81.7 (73.1, 88.4) 1.0000 |
89.3 (84.6, 92.9) 90.0 (85.8, 93.3) 0.8834 |
92.4 (86.1, 96.5) 83.1 (76.9, 88.1) 0.0242 |
|
Month 12b <65 yrs (n=25)
³65 yrs (n=189)
P value |
|
|
77.8 (40.0, 97.2) 91.3 (82.8, 96.4) 0.2247 |
81.3 (54.4, 96.0) 81.7 (73.1, 88.4) 1.0000 |
|
|
|
Month 18c <65 yrs (n=59)
³65 yrs (n=42)
P value |
82.1 (66.5, 92.5) 80.6 (62.5, 92.5) 1.0000 |
95.0 (75.1, 99.9) 81.8 (48.2, 97.7) 0.2814 |
|
|
|
|
|
Month 36c <65 yrs (n=58)
65 yrs (n=39)
P value |
82.1 (66.5, 92.5) 86.2 (68.3, 96.1) 0.7473 |
89.5 (66.9, 98.7) 80.0 (44.4, 97.5) 0.5920 |
|
|
|
|
Table 9 Sensitivity (PPA) and specificity (NPA) from on-site image reads between <65 and ≥65 yrs subjects. Intent to diagnose population
aSummary results calculated across all studies and time points. For Study B, month 12 reference clinical diagnosis was used.
bSummary result calculated for Study B.
cSummary result calculated for Study C.
Sensitivity (PPA)/specificity (NPA) for parkinsonian syndrome (PS) was calculated based on PS present vs. PS absent; Sensitivity (PPA)/specificity (NPA) for dementia with Lewy bodies (DLB) was calculated based on Probably DLB vs. Non-DLB; Sensitivity (PPA)/specificity (NPA) for whole population was calculated based on SDD present vs. SDD absent. P value is from Fisher’s exact test.
CI, confidence interval; n, number of subjects included in the analysis; NPA, negative percent agreement; PPA, positive percent agreement; SDDD, striatal dopaminergic deficit disorder.
|
Reference Clinical Diagnosis |
|||||
|
|
Parkinsonian Syndrome (PS; SDDD) |
Dementia with Lewy Bodies (DLB; SDDD) |
Whole Population |
|||
|
|
Sensitivity % (95% CI) |
Specificity % (95% CI) |
Sensitivity % (95% CI) |
Specificity % (95% CI) |
Sensitivity % (95% CI) |
Specificity % (95% CI) |
|
Mean Resultsa <75 yrs (n=460) ³75 yrs (n=154) P value |
89.2 (85.6, 92.2) 93.0 (80.9, 98.5) 0.6004 |
89.1 (83.3, 93.4) 100 (81.5, 100) 0.2237 |
89.8 (77.8, 96.6) 90.0 (76.3, 97.2) 1.0000 |
78.2 (65.0, 88.2) 84.3 (73.6, 91.9) 0.4864 |
89.3 (86.0, 92.1) 91.6 (83.4, 96.5) 0.6932 |
86.4 (81.1, 90.6) 87.5 (78.7, 93.6) 0.8547 |
|
Month 12b <75 yrs (n=104) ³75 yrs (n=110) P value |
|
|
89.8 (77.8, 96.6) 90.0 (76.3, 97.2) 1.0000 |
78.2 (65.0, 88.2) 84.3 (73.6, 91.9) 0.4864 |
|
|
|
Month 18c <75 yrs (n=92) ³75 yrs (n=9) P value |
81.0 (69.1, 89.8) 85.7 (42.1, 99.6) 1.0000 |
89.7 (72.6, 97.8) 100 (15.8, 100) 1.0000 |
|
|
|
|
|
Month 36c <75 yrs (n=89) ³75 yrs (n=8) P value |
83.9 (72.3, 92.0) 83.3 (35.9, 99.6) 1.0000 |
85.2 (66.3, 95.8) 100 (15.8, 100) 1.0000 |
|
|
|
|
Table 10 Sensitivity (PPA) and specificity (NPA) from on-site image reads between <75 and ≥75 yrs subjects. Intent to diagnose population
aSummary results calculated across all studies and time points. For Study B, month 12 reference clinical diagnosis was used.
bSummary result calculated for Study B.
cSummary result calculated for Study C.
Sensitivity (PPA)/Specificity (NPA) for Parkinsonian Syndrome (PS) was calculated based on PS present vs. PS absent; Sensitivity (PPA)/Specificity (NPA) for Dementia with Lewy Bodies (DLB) was calculated based on Probably DLB vs. Non-DLB; Sensitivity (PPA)/specificity (NPA) for whole population was calculated based on SDD present vs. SDD absent. P value is from Fisher’s exact test.
CI, confidence interval; n, number of subjects included in the analysis; NPA, negative percent agreement; PPA, positive percent agreement; SDDD, striatal dopaminergic deficit disorder.
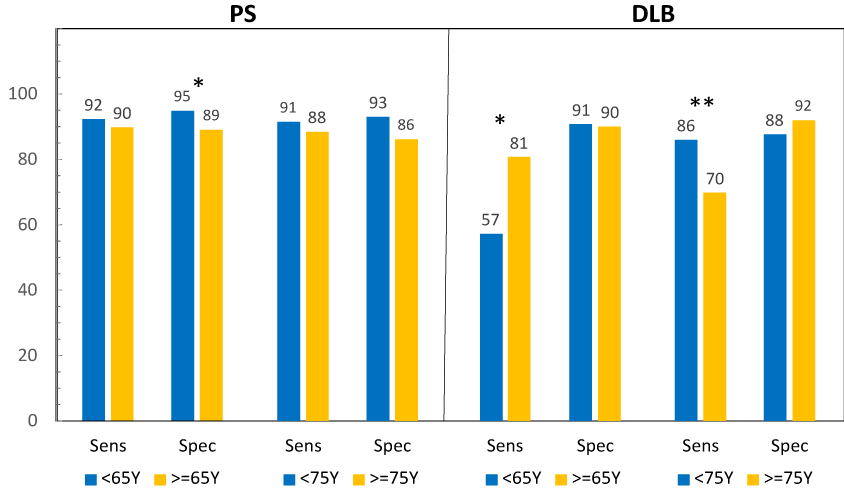
Figure 2 Sensitivity (PPA) and specificity (NPA) in young vs. old subjects with PS and DLB. Sensitivity (PPA) and specificity (NPA), blinded image evaluation reads at baseline in subjects with PS (parkinsonian syndrome) and DLB (dementia with Lewy bodies). *P <0.05; **P <0.01.
Effect of disease state on diagnostic performance
Figure 3 displays sensitivity (PPA) and specificity (NPA) in subjects with PS vs. DLB, broken out by gender and age using both 65-yr and 75-yr cutoffs, using BIE results at baseline. Overall, sensitivity and specificity were numerically lower (not tested for P-value) in subjects with DLB compared with subjects with PS.
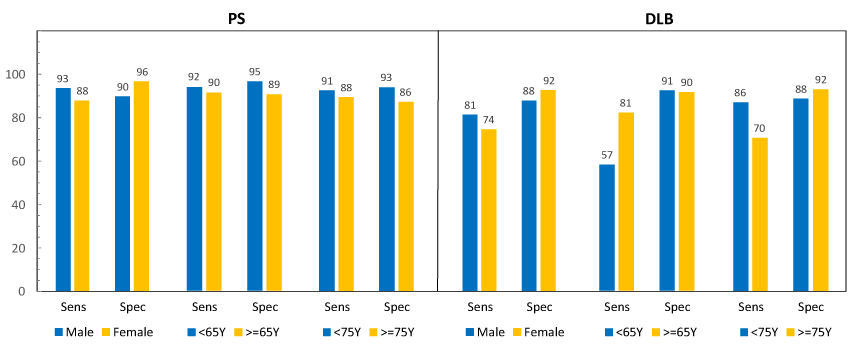
Figure 3 Sensitivity (PPA) and specificity (NPA) in subjects with PS vs. DLB. Sensitivity (PPA) and specificity (NPA), read at baseline in subjects with PS (parkinsonian syndrome) and DLB (dementia with Lewy bodies).
Effects of additional covariates on diagnostic performance
To identify the significant predictors for effects on sensitivity (PPA) and specificity (NPA), a multiple logistic regression model was used. The following covariates were included in the model: disease state (DLB vs. PS), age (as a continuous variable), gender (male vs. female), type of reader (blinded vs. on-site), and duration of follow-up (as a continuous variable). Table 11 summarizes the results for the ITD population. The numbers of observations is the total number of readings from all readers (blinded and on-site) across all time points in all studies (Table 12). For sensitivity, all covariates were significant independent predictors (P<0.05). For specificity, only disease state and type of reader were significant predictors. Results were similar for the PP population and for individual logistical regression for both populations (Tables 12 and 13).
|
|
|
Logistic Regression with All Covariates |
Individual Logistic Regression |
||
|
|
Statistics |
P-value |
Odds ratio (95% CI) |
P-value |
Odds ratio (95% CI) |
|
Sensitivity (PPA) |
|
|
|
|
|
|
Number of Observations |
2485 |
|
|
|
|
|
Number of Observations with True Positive |
2139 (86.1%) |
|
|
|
|
|
Disease state (DLB vs. PS) |
|
0.0028 |
0.642 (0.480, 0.859) |
0.0001 |
0.612 (0.481, 0.780) |
|
Age (yrs)a |
|
0.0086 |
0.982 (0.968, 0.995) |
0.0001 |
0.977 (0.966, 0.989) |
|
Gender (Male vs. Female) |
|
0.0031 |
1.421 (1.126, 1.794) |
0.0030 |
1.412 (1.124, 1.773) |
|
Reading (Blinded vs. On-site)b |
|
0.0016 |
0.622 (0.463, 0.835) |
0.0063 |
0.667 (0.499, 0.892) |
|
Duration of follow-up (months)a |
|
<0.0001 |
0.970 (0.961, 0.979) |
<0.0001 |
0.974 (0.966, 0.982) |
|
Specificity (NPA) |
|
|
|
|
|
|
Number of Observations |
1811 |
|
|
|
|
|
Number of Observations with True Negative |
1632 (90.1%) |
|
|
|
|
|
Disease state (DLB vs. PS) |
|
0.0048 |
0.547 (0.360, 0.832) |
0.0017 |
0.588 (0.423, 0.819) |
|
Age (yrs)a |
|
0.4975 |
1.006 (0.988, 1.024) |
0.1189 |
0.989 (0.975, 1.003) |
|
Gender (Male vs. Female) |
|
0.0839 |
0.754 (0.548, 1.038) |
0.1981 |
0.814 (0.595, 1.114) |
|
Reading (Blinded vs. On-site)b |
|
<0.0001 |
2.307 (1.674, 3.180) |
<0.0001 |
2.322 (1.688, 3.194) |
|
Duration of follow-up (months)a |
|
0.0710 |
1.017 (0.999, 1.037) |
0.0491 |
1.018 (1.000, 1.036) |
Table 11 Logistic regression for ioflupane (123I) sensitivity (PPA) and specificity (NPA) with covariate(s)-ITD population
aAge and duration of follow-up are treated as continuous in the logistic regression model.
bPDT408 only has institutional (on-site) reads.
CI, confidence interval; DLB, dementia with lewy bodies; ITD, intent to diagnose; n, number of subjects included in the analysis; NPA, negative percent agreement; PPA, positive percent agreement; PS, parkinsonian syndrome; yrs, years
|
Study ID |
Reader |
Sensitivity |
Specificity |
|
Study A |
Blinded |
790 |
310 |
|
Study B |
Blinded |
474 |
772 |
|
Study C |
Blinded |
623 |
275 |
|
Study A |
On-site |
158 |
62 |
|
Study B |
On-site |
183 |
271 |
|
Study C |
On-site |
209 |
91 |
|
Study D |
On-site |
48 |
30 |
|
Total |
|
2485 |
1811 |
|
1. ITD Population |
|||
|
Study ID |
Reader |
Sensitivity |
Specificity |
|
Study A |
Blinded |
575 |
210 |
|
Study B |
Blinded |
472 |
767 |
|
Study C |
Blinded |
605 |
275 |
|
Study A |
On-site |
115 |
42 |
|
Study B |
On-site |
173 |
267 |
|
Study C |
On-site |
203 |
91 |
|
Study D |
On-site |
47 |
30 |
|
Total |
|
2190 |
1682 |
|
PP Population |
|||
Table 12 Number of readings, by reader type and study, for sensitivity (PPA) and specificity (NPA)
|
|
|
Logistic Regression with All Covariates |
Individual Logistic Regression |
||
|
|
Statistics |
P-value |
Odds ratio (95% CI) |
P-value |
Odds ratio (95% CI) |
|
Sensitivity (PPA) |
|
|
|
|
|
|
Number of Observations |
2190 |
|
|
|
|
|
Number of Observations with True Positive |
1855 (84.7 %) |
|
|
|
|
|
Disease state (DLB vs. PS) |
|
0.0090 |
0.674 (0.501, 0.906) |
0.0025 |
0.685 (0.537, 0.875) |
|
Age (yrs)a |
|
0.0646 |
0.987 (0.973, 1.001) |
0.0055 |
0.983 (0.972, 0.995) |
|
Gender (Male vs. Female) |
|
0.0017 |
1.463 (1.154, 1.856) |
0.0013 |
1.467 (1.161, 1.853) |
|
Reading (Blinded vs. On-site)b |
|
0.0035 |
0.643 (0.478, 0.865) |
0.0081 |
0.673 (0.502, 0.902) |
|
Duration of follow-up (months)a |
|
< 0.0001 |
0.973 (0.964, 0.983) |
< 0.0001 |
0.977 (0.969, 0.986) |
|
Specificity (NPA) |
|
|
|
|
|
|
Number of Observations |
1682 |
|
|
|
|
|
Number of Observations with True Negative |
1518 (90.2 %) |
|
|
|
|
|
Disease state (DLB vs. PS) |
|
0.0003 |
0.421 (0.264, 0.671) |
0.0008 |
0.536 (0.373, 0.771) |
|
Age (yrs)a |
|
0.0722 |
1.017 (0.998, 1.036) |
0.3755 |
0.993 (0.979, 1.008) |
|
Gender (Male vs. Female) |
|
0.0629 |
0.727 (0.520, 1.017) |
0.1782 |
0.799 (0.576, 1.108) |
|
Reading (Blinded vs. On-site)b |
|
< 0.0001 |
2.628 (1.884, 3.665) |
<0.0001 |
2.609 (1.876, 3.629) |
|
Duration of follow-up (months)a |
|
0.1181 |
1.016 (0.996, 1.036) |
0.0546 |
1.018 (1.000, 1.036) |
Table 13 Logistic regression for ioflupane (123I) sensitivity (PPA) and specificity (NPA) with covariate(s)-PP population
aAge and duration of follow-up are treated as continuous in the logistic regression model.
bPDT408 only has institutional (on-site) reads.
CI, confidence interval; DLB, dementia with lewy bodies; n, number of subjects included in the analysis; NPA, negative percent agreement; PP, per protocol; PPA, positive percent agreement; PS, parkinsonian syndrome; yrs, years
This subgroup analysis is the first to evaluate the effects of gender and age on the diagnostic performance of ioflupane (123I) imaging to detect the presence or absence of an SDD in subjects with a movement disorder or dementia. Pooling the 4 clinical trials provided a large dataset (ITD N =726) to enable this analysis. Although PS and DLB are different disorders, they share the common underlying pathology of striatal dopaminergic deficit. Additionally, there is some overlap in symptomatology, with cognitive impairment observed in patients with PS and DLB patients presenting with motor symptoms. These symptoms accumulate and become more profound with progression of the disease. For this reason, we analyzed the disorders individually as well as the whole population. The BIE image reads which were performed in 3 of the 4 studies, tended to yield more statistically significant differences between subgroups.
Overall, sensitivity and specificity were high in all subgroups analyzed. However, gender had some effect on diagnostic performance in PS and the whole population at baseline when BIE reads were used. Sensitivity was lower and specificity was higher in females compared with males. Studies evaluating gender in PD have shown that differences exist, such as increased prevalence in males (60%), delayed age at onset in females, as well as females more frequently presenting with tremor; for patients presenting with tremor, a slower rate of deterioration was observed 16. Females also had 16% higher ioflupane (123I) binding than males at both the onset of disease and throughout 10 years of follow-up 16. Differences in motor phenotypes have been observed, with males exhibiting symmetrical upper-body disease, whereas females exhibited more postural instability 17. Non-motor phenotypic differences have also been noted, with males having more cognitive impairment, rapid eye movement sleep behavior disorder, orthostatic hypotension, and sexual dysfunction 17. These findings suggest an overall milder course of disease in females, which might explain some of our observations in terms of potential relationships to accuracy of clinical diagnosis, the reference standard used in this study. Higher ioflupane (123I) binding in females may make it more difficult, or at a minimum, delay image interpretation as being abnormal, reducing the sensitivity of detecting an SDDD in the early stages of the disease. This is borne out by the fact that sensitivity in females approximated that in males later in the disease process (month 18 and month 36, Figure 1). On the other hand, because a diagnosis of PD in males is more common (2/3 of all cases) and relatively straightforward, it may be easier to exclude a diagnosis of PD in females (less common; 1/3 of all cases), which would increase specificity. The observed fluctuation in diagnostic performance over time (comparing baseline data with 18 and 36 months) is more likely associated with less precision in the reference standard than in inaccuracies of the interpretation of the SPECT images. This is particularly true, because the 18- and 36-month clinical diagnoses were established based on independent review by movement disorder specialists of videotapes, not by a clinical consensus panel (Study C) 12. Furthermore, the inter-reader agreement, as measured by kappa, was very good/almost perfect 9,12,18,19, which further supports our contention that the observed fluctuations in diagnostic performance were not due to inaccuracies in the interpretations of the SPECT images.
Differences associated with gender in PS were not observed in subjects with DLB, despite recent observations that the incidence of DLB is higher in males 20. This can likely by explained by the smaller sample size of DLB subjects as compared to PS in our analysis, which was also substantiated by Savica et al. 20 by their finding that the overall incidence rate of DLB is lower than that of PD 20.
In spite of all subgroup results being high, age had a slight effect on diagnostic performance, with sensitivity and specificity tending to be lower in older than younger subjects with PS. However, statistically significant differences were only observed for specificity when 65 yrs was used as the cutoff. Sensitivity was also lower in the whole population, reaching statistical significance using both age cutoffs, whereas specificity was only statistically significantly lower in older subjects when the 65-yr cutoff was used. This may be explained by increasing presence of multiple comorbid conditions with advanced age that could complicate clinical diagnosis. Additionally, older individuals may have mixed pathologies that make clinical diagnosis more challenging. Lastly, the decreased binding of ioflupane (123I) with normal aging 3-6 could make it more difficult to discriminate normal from abnormal images later in life.
Clinical diagnosis had an effect on the diagnostic performance of ioflupane (123I) imaging. Overall, even though the mean values for sensitivity and specificity in the whole population were high, it tended to be slightly lower in subjects with DLB compared with subjects with PS (statistical difference was not tested). This can most likely be explained by the fact that dementia subtype is more difficult to diagnose than PS, and the reduced accuracy can be attributed to less precision in the reference standard (i.e., the clinical diagnosis of DLB 21 or other dementia subtypes (with or without SDD), rather than inaccuracies in the interpretations of the SPECT images.
When a multiple logistic regression model was used, all covariates (disease state DLB vs. PS), age, gender (male vs. female), type of reader (blinded vs. on-site) and duration of follow-up) were significant predictors of the model effect on sensitivity. For specificity, only disease state and type of reader were significant predictors. The observation that age and gender did not affect specificity in the multiple logistic regression model is important and supports the robust performance and high accuracy of this diagnostic test. Age and gender had a statistically significant effect on sensitivity, which may be explained by the points raised earlier. Additional research may be needed to further delineate whether the effects observed are reproducible and clinically relevant.
This subgroup analysis had some limitations. This was an exploratory analysis, and as such, unadjusted p-values were calculated. If corrections for multiple comparisons had been made, the results may have been different. Image assessments were only performed visually. If quantification procedures had been included, differences may have been observed in the sensitivity and specificity results. Although clinical diagnosis is considered a valuable reference standard in developing radiopharmaceuticals for movement disorders and dementia 22, the definitive truth standard is neuropathological confirmation of brain tissue at autopsy. However, it is impractical to design clinical trials to span the life-expectancy of the study subjects, and so clinical diagnosis is the best alternative. For this reason, in our paper, in addition to sensitivity and specificity, we also used the terms PPA and NPA (mathematically identical calculations, but emphasizing our limitations of not being the truth standard). Despite taking measures to minimize errors in clinical diagnosis, such as using panels of experts, inaccuracies likely occurred, and may be were responsible for the reductions observed in sensitivity and specificity. Additionally, age and gender had some statistically significant effects on the diagnostic performance of ioflupane (123I) imaging, although to what extent these differences are clinically relevant is unknown. The observed effects should be taken into consideration and allowed for when assessing patients presenting with varying diagnostic states.
This subgroup analysis showed that gender and age have some effect on the diagnostic performance of ioflupane (123I) imaging in subjects with movement disorders or dementia when clinical diagnosis is used as the reference standard. Overall, sensitivity and specificity were high. Statistically significant differences were observed in some comparisons. Sensitivity was slightly reduced, though still diagnostically useful, above the age of 75. In subjects with PS, sensitivity was higher in males, whereas specificity was higher in females. Multiple logistic regression model analysis demonstrated that all tested covariates (including age and gender) were significant predictors for sensitivity, but not for specificity, where only disease state and type of reader were significant predictors. To what extent these differences are clinically relevant remains to be elucidated. Additional research may provide further clarification on these issues.
DG Grosset: Received grants and personal fees from Merz Pharma; and personal fees from Astellas, Civitas, InVentiv Health, AbbVie, GE Healthcare, Teva and UCB Pharma.
JT O’Brien: Received grants, lecture fees, and has provided consultancy to GE Healthcare and Lilly; received lecture fees and provided consultancy to Bayer Healthcare; and provided consultancy to TauRx and Cytox.
WH Oertel: Received grants and personal fees from GE Healthcare for performing DaTscan clinical trials, educational presentations, and reimbursement of travel costs; and personal fees from Amersham Buchler for medical and scientific expert testimony at EMEA for DaTscan.
IG McKeith: Received grants and personal fees for speakers’ bureaus and consultancy from GE Healthcare.
Z Walker: Received grants and personal fees for research, consulting, speakers’ bureaus, lectures, and travel expenses from GE Healthcare; personal fees for consultancy from Bayer Healthcare and Novartis; and grants from Lundbeck.
K Tatsch: Received personal fees for performing DaTscan SPECT image reads during clinical trials and honoraria for lectures from GE Healthcare.
E Tolosa: Received personal fees for research, lectures and consultancy from Novarits, TEVA, Boehringer Ingelheim, UCB, Solvay, and Lundbeck; grants from the Spaniard Network, Michael J. Fox Foundation, and Fondo de Investigaciones Sanitarias de la Seguridad Social.
PF Sherwin: Full-time employee of GE Healthcare.
ID Grachev: Full-time employee of GE Healthcare at the time of the study and does not hold any stocks or shares, or have other financial competing interests.
We thank Stacy Simpson Logan, CMPP of Winfield Consulting, who provided medical writing services, funded by GE Healthcare. This study was financially supported by GE Healthcare. GE Healthcare Medical Director (IDG) jointly with the study team and co-authors contributed to the design of the study, and the collection, analysis, and interpretation of the data. The authors were responsible for the decision to submit the manuscript for publication.

©2014 Grosset, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.