Journal of
eISSN: 2373-6410


Review Article Volume 14 Issue 5
1European Wellness Biomedical Group, Germany
2Baden R&D Laboratories, Germany
3FCTI Biotech R&D, Germany
Correspondence: Prof. Dr. Dmytro Klokol, MD, PhD, Head of Medical Advisory, EWBG,
Received: August 24, 2024 | Published: September 2, 2024
Citation: Klokol D, Chan MTKS, Wong MBF. Advancements in autism: exploring innovative therapies and targeted approaches to management and reversal of neurodevelopmental disorders. J Neurol Stroke. 2024;14(5):122-132. DOI: 10.15406/jnsk.2024.14.00594
This article explores effective treatment strategies for Autism Spectrum Disorders (ASD), focusing on innovative targeted therapies within the field of regenerative medicine. We reviewed and assessed the use of advanced regenerative techniques in treating ASD, highlighting new therapeutic modalities that address key pathological pathways associated with autism. The discussion includes cellular therapies, neuropeptide interventions, and treatments involving neuromediator precursors. Clinical outcomes were measured by improvements in behavioral, cognitive, and social functions. Patients with autism who received comprehensive biological therapy showed noticeable improvements in self-regulation and behavior within the initial weeks of treatment. By 2-3 months, significant progress was observed in eye contact, attention span, verbal responsiveness, and speech development. Follow-ups at six months indicated better communication skills and self-management. The addition of neuropeptides during the maintenance phase led to marked reductions in anxiety and aggression, and improved social skills and communication. Long-term therapy demonstrated sustained enhancements in conversational abilities, decreased repetitive behaviors, and improved social and emotional interactions. The combination of novel biological therapies and nutraceuticals appears to offer a promising approach to managing ASD, with significant gains in behavioral and cognitive outcomes. These results suggest the potential of regenerative medicine in improving ASD treatment and highlight the need for further research to refine treatment protocols and confirm long-term benefits.
Keywords: autism, autism spectrum disorders, stem cells, regenerative medicine, neurodevelopmental disorders, neuropeptides, behavioral therapy, cognitive development, social skills
Autism Spectrum Disorders (ASD) encompass a range of neurodevelopmental conditions marked by challenges in social interaction and communication, as well as atypical behavioral patterns. These can include difficulties transitioning between activities, a focus on specific details, and unusual responses to sensory experiences. Typically identified in early childhood, ASD can significantly affect educational and employment prospects, requiring families and caregivers to offer substantial support and care. Individuals with ASD commonly exhibit varying degrees of difficulty in social communication and interaction, alongside repetitive, restricted, and stereotyped behaviors.1
Persistent deficits in social communication and social interaction include: making little or inconsistent eye contact; tending not to look at or listen to people; rarely sharing enjoyment of objects or activities by pointing or showing things to others; failing to, or being slow to, respond to someone calling their name or to other verbal attempts to gain attention; having difficulties with the back and forth of conversation; often talking at length about a favorite subject without noticing that others are not interested or without giving others a chance to respond; having facial expressions, movements, and gestures that do not match what is being said; having an unusual tone of voice that may sound sing-song or flat and robot-like; having trouble understanding another person’s point of view or being unable to predict or understand other people’s actions. Restricted, repetitive patterns of behavior, interests, or activities: repeating certain behaviors or having unusual behaviors; having a lasting intense interest in certain topics, such as numbers, details, or facts; having overly focused interests, such as with moving objects or parts of objects; getting upset by slight changes in a routine; being more or less sensitive than other people to sensory input, such as light, noise, clothing, or temperature.1–5
According to the WHO the prevalence of ASD worldwide is 1 in 100 children.2 Most of the studies show a dramatic increase in ASD prevalence since the late 1900s and early 2000s. According to data from the Autism and Developmental Disabilities Monitoring (ADDM) Network, established by the Centers for Disease Control and Prevention (CDC) in USA, the prevalence of ASD among 8-year-old children in the United States has increased from 67 per 10,000 in 2000 to 90 per 10,000 in 2006 and 145 per 10,000 in 2012.3–5 The latest report from 2018 indicates that approximately 230 per 10,000 children are affected by ASD, reflecting a more than 200% increase in prevalence since the initial ADDM Network study in 2000.6 A systematic review that analyzed 71 studies and 99 prevalence estimates from 34 countries, covering publications from 2012 to 2021, indicated a median prevalence of 100 per 10,000 children.7 In contrast, the meta-analysis by Salari et al., which included 74 studies published between 2008 and 2021, reported a pooled prevalence of 60 per 10,000.8
Despite the high prevalence of Autism Spectrum Disorders (ASD), there are no universally standardized or guaranteed treatment strategies for these conditions. The Centers for Disease Control and Prevention (CDC) outlines a range of current treatment options, including behavioral, developmental, educational, psychological, social-relational, pharmacological, and complementary and alternative therapies. Developmental approaches focus on improving specific skills, such as language or motor abilities, or a broader set of related developmental functions. These are often combined with behavioral strategies. Among developmental therapies, speech and language therapy is particularly common for individuals with ASD. It aims to enhance communication skills, including both the understanding and use of speech and language. While some individuals with ASD communicate verbally, others may use alternative methods such as signs, gestures, pictures, or electronic devices. Occupational therapy is intended to foster independence by teaching essential daily living skills, such as dressing, eating, and bathing, and may include sensory integration therapy to address issues with sensory processing. Physical therapy focuses on improving physical abilities, such as fine motor skills and larger body movements.
Pharmaceutical treatments are designed to manage co-occurring symptoms that accompany ASD and can improve functioning. For example, medications may address difficulties with concentration or behaviors such as head banging and hand biting. They can also help alleviate associated conditions like anxiety, depression, seizures, sleep disturbances, or gastrointestinal issues. Complementary and alternative therapies are often used in conjunction with traditional treatments to provide comprehensive care. These may include specialized diets, herbal supplements, chiropractic care, animal-assisted therapy, art therapy, mindfulness practices, or relaxation techniques. It is important for individuals and families to consult with healthcare providers before starting any complementary or alternative treatments.
Despite the growing prevalence of ASD and increased focus on the condition, there are no universally accepted treatment strategies that guarantee significant improvement. In our previous research, we have explored various aspects of the etiology, pathogenesis, symptomatology, and treatment options for ASD.9,10 The goals and objectives of this article are to provide a profound analysis of neuroanatomy of the ASD, discuss its pathogenesis and metabolic disruptions occurring in the ASD brain and provide the high-efficacy pathogenetically validated treatment protocols for the spectrum disorders.
Let us examine the anatomical distinctions noted between brains affected by ASD and those of neurotypical individuals. Research has frequently shown that ASD is associated with an enlargement of the frontal cortex in early childhood. Other studies have highlighted differences in the size of various brain regions and potential epigenetic changes, which may indicate differences in brain function. At the cellular level, ASD has been linked to cortical dysgenesis, including variations in cortical thickness, neuronal density, and minicolumn organization, as well as localized disorganization. These findings of atypical brain structure have renewed interest in understanding the fundamental mechanisms that influence brain development.11 Difficulties with social interaction, reward prediction, emotional memory, and facial and emotional recognition in ASD may reflect dysfunction in the amygdala and its connected regions.12,13 Recent research has investigated how abnormal growth patterns of the amygdala affect children and adolescents with ASD.14
The abnormalities in the amygdala in individuals with autism involves increased cell densities and smaller neuronal cell sizes.15 However, the findings of that study were based on a limited sample of only six cases, including four with comorbid seizure conditions, which may have influenced the observed anatomical changes. Schumann identified alterations in the relative size of the amygdala in young children. An MRI study involving 89 children aged 1 to 5 years found that toddlers diagnosed with ASD had larger right and left amygdalae compared to typically developing peers, even when adjusted for total brain volume.16 Notably, the degree of amygdala enlargement in these children correlated positively with the severity of social interaction and communication challenges observed by age of five years old.
Other research has also reported increased amygdala volume in autistic individuals under the age of 5 years.17,18 Conversely, another study found no evidence of amygdala enlargement in adolescents with ASD, suggesting that changes in amygdala size and cell count may be age-dependent.19 The prevailing view is that while the amygdala may enlarge early in life for those with ASD, this enlargement typically slows and may even reverse, resulting in a smaller amygdala with fewer neurons in adults with ASD compared to controls. The cerebellum is traditionally recognized for its function in proprioception and fine motor control. However, recent studies have increasingly emphasized its role in higher cognitive functions, such as language processing, cognitive tasks, and emotional regulation.20–22
Some studies found hypoplasia of the central cerebellar vermis lobules as the first neuroanatomical shifts in ASD.23 Numerous studies have documented cerebellar abnormalities in ASD.22. A meta-analysis, which examined 17 voxel-based morphometry studies of grey matter volume, compared the cerebella of male ASD patients to age-matched neurotypical controls.24 Yet, these findings are not universally observed across all studies or cohorts. For instance, some research has reported no changes in cerebellar volume or cerebellar hyperplasia (Table 1).25,26
|
Social impairment |
Communication deficits |
Repetitive behaviours |
|
OFC – Orbitofrontal cortex ACC – Anterior cingulate cortex FG – Fusiform gyrus STS – Superior temporal sulcus A – Amygdala mirror neurons IFG – Inferior frontal gyrus PPC – Posterior parietal cortex |
IFG – Inferior frontal gyrus (Broca’s area) STS – Superior temporal sulcus SMA – Supplementary motor area BG – Basal ganglia SN – Substantia nigra Th – Thalamus PNC – Pontine nuclei cerebellum |
OFC – Orbitofrontal cortex ACC – Anterior cingulate cortex BG – Basal ganglia Th – Thalamus |
Table 1 Links between the neuroanatomy and clinical manifestations of ASD
A notable and consistently observed neuroanatomical abnormality in postmortem brains of individuals with ASD is a significant reduction in both the size and number of Purkinje cells, especially within the posterolateral neocerebellar and archicerebellar cortices.27 Given that Purkinje cells are the only output neurons of the cerebellum, their reduction could have substantial functional consequences. Analysis of data from 24 postmortem studies indicated a significant 79% prevalence of decreased Purkinje cell numbers in the cerebellar hemispheres of ASD brains.28 A stereological assessment of Purkinje cell size and morphology in ASD showed a 25% reduction in the total number of Purkinje cells and a 24% decrease in cell density in the cerebella of 14 individuals with ASD compared to control subjects.29 Since Purkinje cells are the sole output neurons of the cerebellum, their reduction may have significant functional implications. Another study reported significant reductions in Purkinje cell volume, approximately 31% in 4- to 8-year-olds and around 23% in individuals aged 29 to 60.30
The frontal cortex plays a vital role in executive functions, encompassing complex cognitive tasks such as decision-making, planning, working memory, emotional regulation, social behavior, learning, and communication. Due to the social and emotional difficulties associated with ASD, this brain region has garnered significant research attention. Key findings in ASD research highlight unusual patterns of cortical growth, irregular cortical thickness, and disorganized neuronal arrangements across cortical layers and their connections with other brain regions. Previous MRI studies have indicated that children with ASD often exhibit atypical growth patterns, including early excessive growth of the frontal cortex.31 The analysis of MRI scans of 41 toddlers with diagnosed ASD and 44 typically developing controls across various time points revealed a notable 7% increase in overall cerebrum size in autistic toddlers compared to controls up to 2.5 years of age, with a 10% rise in white matter and a 5% increase in grey matter.32
Top of Form
Bottom of Form
Recent findings from a large-scale study are consistent with earlier research, showing an increase in cortical thickness during early childhood, which is followed by a deceleration in growth and a cessation of development during later childhood and adolescence.33 This study observed that while cortical thickness tends to normalize between ages 8 and 18, this normalization might not indicate broader structural or functional improvements. In the neocortex, neuronal cell bodies are organized into minicolumns, stacked one above the other. Analysis of the microstructure in layer III of the dorsolateral prefrontal cortex and Brodmann area from 14 postmortem ASD samples revealed a reduction in the spacing between minicolumns.34–36 The study of McKavanagh et al. observed wider minicolumns in the primary auditory, auditory association, orbital frontal, and parietal cortices of ASD brains, especially in younger individuals.37 These findings further support the idea of an atypical developmental trajectory in ASD.
A study analyzing postmortem tissue from the prefrontal, temporal, and occipital neocortices of 11 children with ASD and 11 typically developing controls, aged 2 to 15 years, utilized in situ hybridization on tissue sections to investigate gene expression patterns. The findings revealed patches of altered or reduced gene expression, ranging from 5 to 7 mm in size, in 10 out of 11 ASD cases, compared to only 1 out of 11 control cases.38 These disruptions may result from abnormalities in neuronal proliferation, differentiation, migration, and/or survival, leading to improper neuronal positioning within cortical layers (Figure 1).
In addition to macro- and microanatomical changes, the brain in individuals with autism exhibits greater functional hyperconnectivity compared to typically developing brains. Supekar et al. conducted a study using data from 110 children collected across three different sites, making it the largest pediatric brain imaging dataset available.39 The findings provided robust and consistent evidence of widespread functional hyperconnectivity in children with ASD. This hyperconnectivity is validated not only by the study's significant triple-replication but also by its correlation with clinical symptoms of ASD. Specifically, children with more severe social impairments demonstrated increased functional connectivity. This research is the first to identify a significant link between atypical whole-brain functional connectivity and a core symptom of ASD.
A study of children aged 7 to 13 found that those with ASD exhibited increased connectivity within striatal systems compared to their typically developing peers. This finding of "ectopic" hyperconnectivity in a specific brain system aligns with and expands upon our current research by providing a whole-brain perspective for the first time.40 The analysis of functional subsystems identified hyperconnectivity across various regions, including sensory and association cortices. These results underscore abnormal connectivity patterns in brain systems related to cognitive, social, and emotional functions.41 Examining connectivity patterns relative to anatomical distance revealed hyperconnectivity in ASD between both adjacent and distant brain regions, suggesting atypical integration and segregation in short- and long-range circuits.39 Overall, these findings provide new insights into the extensive functional brain hyperconnectivity in childhood ASD, impacting both global brain connectivity and major functional subsystems across different anatomical distances.
Both genetic and environmental factors are thought to significantly contribute to the development of ASD. Recently, there has been heightened attention on the effects of exposure to neurotoxic substances. These substances, whether of natural or synthetic origin, can disrupt nervous system function. Many everyday products contain chemicals that are suspected of having harmful effects on neurodevelopment. Consequently, there is increasing concern about the impact of these neurotoxic compounds, which can be found in both natural and manufactured sources, on the nervous system and overall neurodevelopment.
Thus, one of the studies has linked acetaminophen use in children to an increased risk of developing autism. A parents’ survey found an association between acetaminophen use following measles-mumps-rubella (MMR) vaccination and autistic behaviors in children up to 5 years old.42 Animal studies have shown that acetaminophen exposure can affect social behavior in mice.43 It was suggested that acetaminophen might influence the endocannabinoid system, potentially leading to neuromodulatory effects during development that could trigger autism.44 Hence, cannabinoid receptor type 2 has been found to be upregulated in the blood cells of children with ASD.45
During the first months of life, the human brain evolves from a simple cell layer into a sophisticated organ with billions of specialized, intricately connected cells.46,47 Therefore, exposure to neurotoxic compounds can be especially detrimental to brain development during this critical phase. However, brain development does not stop at birth. The growth of glial cells and the myelination of axons continue for several years, while synaptic development persists through childhood and adolescence, extending the period of susceptibility to neurotoxic agents.47,48 Disruptions in neurotransmitter function may play a critical role, as research has connected neurotoxic substances to changes in neurotransmitter activity, which are subsequently linked to ASD. Various neurotransmitters, such as γ-aminobutyric acid (GABA), glutamate (Glu), serotonin (5-HT), and dopamine (DA), have been associated with ASD.27 Changes in both the levels of these neurotransmitters and their associated proteins - such as receptors and transporters - have been connected to the disorder. Disruptions in acetylcholine neurotransmission caused by organophosphate exposure may also affect other neurotransmitter systems.49 Additionally, organochlorine pesticides have been associated with the inhibition of GABAA receptors.50 Specifically, the organochlorine pesticide endosulfan interferes with the binding of GABA to its receptor site, resulting in excessive neuronal excitation.
The potential role of mercury in neurotoxicity and the development of ASD has been widely debated. Earlier research identified significantly higher hair mercury levels in children with autism compared to age- and sex-matched healthy controls.51 These findings are consistent with other studies that also reported elevated mercury levels in the hair of autistic children relative to their non-autistic peers. Furthermore, Adams et al. observed that the mechanisms for excreting toxic metals might differ notably between individuals with moderate to severe ASD and those with milder forms of the disorder.52 Children are particularly susceptible to environmental toxins due to their higher absorption rates and lower detoxification capacities compared to adults. Mercury is especially concerning for its neurotoxic effects, which can significantly impact neurodevelopment. Moreover, simultaneous exposure to multiple toxic elements may exacerbate neurotoxicity, complicating efforts to isolate the effects of individual elements on ASD.53 Thiomersal, a mercury-based preservative found in certain vaccines like the Measles-Mumps-Rubella (MMR) vaccine, has long been scrutinized for its potential link to autism. As an antiseptic and antifungal agent, thiomersal's mercury content has raised concerns about its role in autism. In autistic children, exposure to toxic substances is believed to be connected to oxidative stress, impaired methylation and transsulfuration pathways, and mitochondrial dysfunction.54
A comprehensive meta-analysis reviewed 18 studies on aluminium (Al), 18 on cadmium (Cd), and 23 on mercury (Hg) in individuals with ASD. While the analysis found significant associations between all three metals and ASD, the nature of these associations varied. Specifically, elevated mercury levels in hair, urine, and blood were consistently associated with ASD. In contrast, aluminium levels in hair and urine were positively correlated with ASD, whereas aluminium levels in blood showed a negative correlation. For cadmium, higher levels in hair and urine were inversely related to ASD. These results indicate that although these metals are all neurotoxic, their impact on ASD and their mechanisms of action may differ. Further research is necessary to understand the long-term effects of these toxic metals on ASD risk, identify critical periods of exposure, and explore factors that may influence their impact. Overall, the findings support the implementation of policies to reduce exposure to neurotoxic metals, particularly for pregnant women and young children, in order to help address the rising prevalence of ASD.55
Another study involving one hundred children with ASD in comparison to a hundred of control non-ASD individuals showed the mean levels of mercury, lead, and aluminium in hair of the autistic children were significantly higher than controls.56 These findings allowed researchers to conclude that environmental exposure to these toxic heavy metals, at key times in development, may play a causal role in autism (Figure 2 & 3).
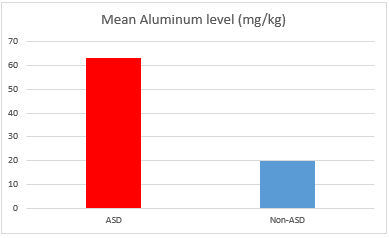
Figure 2 Mean Aluminum level in the hair of ASD vs non-ASD individuals (from: Mohamed Fel B. et al. Assessment of Hair Aluminum, Lead, and Mercury in a Sample of Autistic Egyptian Children: Environmental Risk Factors of Heavy Metals in Autism. Behav Neurol. 2015.).
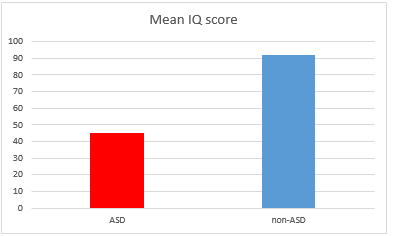
Figure 3 Mean IQ score in ASD vs non-ASD individuals in the same cohorts corresponding to the Aluminum levels in hair (from Mohamed Fel B. et al. Assessment of Hair Aluminum, Lead, and Mercury in a Sample of Autistic Egyptian Children: Environmental Risk Factors of Heavy Metals in Autism. Behav Neurol. 2015).
Excitotoxicity is a pathological state characterized by excessive neuronal activation resulting from the overactivation of excitatory amino acid receptors, such as those for glutamate and aspartate.57 Morphologically-speaking it is characterized by neuronal swelling and accelerated cellular death.58 Excitatory receptors allow the movement of sodium, calcium, and potassium ions, which leads to neuronal activation.59 The elevated levels of glutamate and other excitatory substances can lead to excessive activation of ionotropic glutamatergic receptors. This excessive activation results in elevated intracellular calcium levels. The increase in calcium triggers the activation of inducible nitric oxide synthase and protein kinase C, which further amplifies nitric oxide production and activates phospholipase. This process generates pro-inflammatory molecules.60,61 The resulting free radicals can impair oxidative phosphorylation and damage mitochondrial enzymes involved in the electron transport chain, reducing energy production.62 In autistic individuals the excitotoxicity can be also associated with seizures. Evidence from experimental studies supports a link between seizures and excitotoxicity, with elevated brain glutamate levels being a potential trigger for seizures.63,64 Additionally, immune responses triggered by microglial activation and cytokine release contribute to excitotoxicity.65
Biochemical assessments have revealed higher serum levels of glutamate in individuals with autism compared to controls.66 Research has also linked disruptions in immune system regulation with autism.67 Additionally, increased levels of tumor necrosis factor-alpha (TNF-alpha) receptor II have been noted in the blood of children with autism spectrum disorders.68 During immune responses, microglial activation leads to the release of TNF-alpha, which exacerbates excitotoxicity by boosting reactive oxygen and nitrogen species and further impeding the reuptake of glutamate.69 Furthermore, activated microglia can release potent excitotoxins, including glutamate and quinolinic acid.70 Research has indicated a connection between the development of seizures and excitotoxicity, particularly involving glutamate accumulation.71 Seizures can lead to the production of excitotoxic amino acids by increasing the generation of free radicals. Importantly, the developing brain in infants is especially susceptible to excitotoxic damage due to a higher density of synaptic glutamate receptors compared to newborns, with this receptor density gradually decreasing with age.72
The overactivation of the glutamate receptors leads to the release of additional excitotoxins and an increase in glutamate levels.73 High concentrations of glutamate cause elevated calcium levels within the cytosol. This occurs because excessive glutamate prolongs the opening of calcium channels, resulting in an increased influx of calcium into cells. The rise in calcium levels activates inducible nitric oxide synthase and protein kinase C, which in turn generate free radicals, ROS, and arachidonic acid. The production of these oxidants can impair mitochondrial function, lead to the buildup of pro-inflammatory molecules, and ultimately result in cell death.
Gamma-aminobutyric acid (GABA), being the principal inhibitory neurotransmitter, and its reduced inhibitory function is one of the many abnormalities seen in ASD. When GABAergic inhibition is diminished, it can lead to an overabundance of glutamate activity in neurons and impaired sensory gating. This suppression may occur through the direct dysfunction of GABA receptors or through the antagonism of GABAergic neurons that have receptors sensitive to the glutamate analog NMDA.74 Additionally, excessive activation of non-NMDA glutamatergic receptors can lead to a reduction in the number of synapses and limit dendritic growth in pyramidal neurons within the hippocampus, which is the primary site of neuronal precursors’ production.
Calcium ions (Ca2+) play a critical role in mediating excitotoxic damage within cells. They are vital for various cellular functions, including membrane excitability, cell growth, exocytosis, and synaptic activity. Under normal conditions, neurons employ specialized mechanisms to regulate cytosolic Ca2+ levels and keep calcium concentrations low.However, excessive release of glutamate from synapses and overstimulation of glutamate receptors, such as NMDA, AMPA, and kainate receptors, can lead to excessive receptor activation. This overstimulation opens associated ion channels, causing an increased influx of calcium and sodium ions. This disruption in calcium regulation can ultimately result in cell death.75 Genetic factors also contribute significantly to excitotoxicity in ASD. Mutations in genes that encode voltage-gated calcium channels can be linked to AS. These channels, present in dendrites and cell bodies, play a key role in regulating neuronal excitability and calcium-dependent signaling pathways.76,77 Mutations can cause these channels to remain open longer than normal, leading to excessive calcium influx (Figure 4).78,79
The brain has exceptionally high energy demands, consuming approximately 20% of the body's total caloric intake while accounting for only about 2% of body weight. It requires significant amounts of adenosine triphosphate (ATP) to maintain ionic gradients essential for neurotransmission and neural plasticity.80 Mitochondria are pivotal in various aspects of neural development and function, including the proliferation, differentiation, and maturation of neural stem cells. They also play a critical role in the growth of dendritic processes and are involved in both developmental and synaptic plasticity. Additionally, mitochondria influence cell survival and apoptosis.81–84
Disruptions in folate metabolism have been linked to ASD. Variations in genes related to folate metabolism may elevate the risk of developing ASD through complex polygenic interactions. Furthermore, autoantibodies that obstruct folate transport into the brain have been associated with ASD, and high doses of folinic acid may mitigate the effects of these antibodies. Similar metabolic disturbances are observed in mothers of children with ASD, and folate supplementation before conception and during pregnancy has been shown to reduce the risk of ASD in offspring. These observations suggest that abnormalities in the folate pathway could be a significant metabolic factor in ASD and may aid in developing biomarkers for improving symptoms and potentially preventing the disorder.85
Recently there has been growing evidence of mitochondrial dysfunction in ASD, with abnormal lactate and pyruvate levels frequently reported. Additionally, oxidative stress has been consistently documented in children with ASD through various experimental methods. Research has also highlighted biomarkers of vitamin deficiency, impaired energy transport, reduced sulfation, and detoxification in these individuals. Abnormal fatty acid metabolism has been noted in some cases of ASD. Besides that, the imbalances in gut microbiota and their potential link to gastrointestinal issues in ASD have gained attention. Overgrowth of specific gut bacteria has been reported, with studies showing increased levels of certain Clostridium species in the feces of autistic children compared to controls.86 Thus, a study comparing 13 children with autism and 8 controls found that nine Clostridium species were present in the feces of the autistic children but not in the controls. Increased prevalence of Clostridium histolyticum and associations between high levels of Sutterella species and gastrointestinal disturbances in children with autism have been reported.
Neuroinflammation involves the interaction of neurons, microglia, and macroglia within the CNS.87,88 This inflammatory response is a common feature in various neurodegenerative disorders, including multiple sclerosis (MS), Alzheimer's disease (AD), Parkinson's disease (PD), and neurodevelopmental disorder autism.88,89 Individuals with ASD often display altered inflammatory responses in the CNS and the immune system throughout their lives, indicating that inflammation may contribute to the development of ASD. Growing clinical and experimental evidence supports this notion, showing a connection between immune system irregularities and the pathogenesis of ASD.90 Additionally, post-mortem analyses have revealed significant neuroinflammation in various brain regions of individuals with ASD.91
Defects in microglia have been linked to ASD as well.88,89 When activated, microglia can lead to neuronal dysfunction and cell death, exhibiting neurodegenerative effects. Activated microglia display various biological responses, such as cell rounding, proliferation, migration, phagocytosis, antigen presentation to T-cells, and the release of reactive oxygen species. In ASD, chronic or excessive neuroinflammation has been observed, with ongoing glial activation and inflammatory dysfunction potentially playing a role in the behavioral symptoms associated with autism.92 Chronic peripheral inflammation and abnormal brain inflammatory responses may lead to cognitive impairments.90
Activated microglia can induce neuronal dysfunction and cell death, leading to neurodegenerative effects. These microglia exhibit several biological responses, including cell rounding, proliferation, migration, phagocytosis, antigen presentation to T-cells, and the release of reactive oxygen species. In the context of ASD, persistent or excessive neuroinflammation has been noted, with continuous glial activation and inflammatory dysfunction potentially contributing to the behavioral symptoms of autism.9. Significant benefits have been achieved through integration of novel therapeutic modalities with biological agents targeting the intrinsic subcellular biochemical pathways leading to ASD. The holistic paradigms of therapeutic correction of neurodevelopmental disorders include multidimensional therapeutic modalities ranging from nutritional corrections, nutraceuticals, orthomolecular therapy, application of energy devices stimulating neurodevelopment and neuroplasticity, brain engagement, neuropeptides, and Stem cell therapy with precursor stem cells (PSC), which are effective not only in early childhood but also in adults with autism, who were not treated timely in the past. During neurogenesis neural progenitor cells express regulatory genes that subdivide each area of the brain into compartments and regulate their size. Progenitor cells generate neurons during early to mid-embryogenesis. Neurons increasingly migrate to their target sites sharing the transcription factors expressed by neuronal progenitors.93,94
The approach to diagnosing ASD typically involves comprehensive diagnostic measures, including biochemical laboratory tests, allergy and food intolerance assessments, functional evaluations, and ASD scoring. Regular, long-term reassessments of key biochemical, metabolic, and functional parameters are essential to monitor progress effectively. Various scoring systems are employed for this purpose, such as the Childhood Autism Rating Scale (CARS), Gilliam Autism Rating Scale (GARS), Autism Treatment Evaluation Checklist (ATEC), Vineland Adaptive Behavior Scales (VABS), Clinical Global Impression Scales for Severity (CGI-S) and Global Improvement (CGI-I), Pervasive Developmental Disorder Behavior Inventory (PDDBI), and the European Wellness Functional Assessment Score, among others.
Addressing nutritional needs by supplementing deficiencies in macro and micronutrients commonly associated with ASD is crucial, while avoiding nutrients and toxins that might worsen symptoms. For instance, sugars, MSG, casein, and gluten can contribute to intolerances or exacerbate ASD symptoms and are therefore recommended for exclusion from the diet. Vitamin D3 and Omega-3 fatty acids have been shown to help reduce hyperactivity and irritability. Additionally, folic acid supplementation can improve social, behavioral, and cognitive functions. Maternal folic acid supplementation has also been linked to a decreased risk of ASD in offspring.95 Such clinical symptoms as irritability, aggression, as well as some of the metabolic dysfunctions can be effectively addressed with supplementation of vitamin B12. Vitamin B6 and Magnesium are supplemented in adequate doses according to the child’s age and bodyweight and are essential micronutrients involved in speech development and relaxation of the CNS.
Prebiotics are stimulating the growth of beneficial gut microbiota, and upon degradation by the gut microbiota, produce increased level of circulating short-chain fatty acids.96 Probiotics Bifidobacterium infantis and bovine colostrum improved overall aberrant behaviors and gastrointestinal disorders, and decreased levels of circulating inflammatory cytokines IL-13 and TNF-α.97 Gamma-aminobutyric acid (GABA) serves as the main inhibitory neurotransmitter in the central nervous system. Due to its main function to “diminish neuronal excitability across the nervous system”, by lowering overall neural activity, GABA helps to mitigate responses associated with the fight-or-flight reaction, including fear, anxiety, aggression, stress, and agitation. Few studies have reported a correlation between abnormal melatonin concentrations and the severity of ASD symptoms.98 Dozens of clinical trials have reported improvements in sleep parameters with exogenous melatonin supplementation in ASD, including longer sleep duration, less nighttime awakenings and quicker sleep onset.99. In our observations the melatonin supplementation in ASD brings significant improvements in sleep duration, sleep onset latency, and promotes positive changes in daytime behaviors.
Phosphatidylcholine plays a crucial role in maintaining healthy brain function in individuals on the autism spectrum. Autism is associated with reduced levels of phosphatidylcholine in the brain tissue, which lead to various cognitive and behavioral symptoms. In particular, it is the choline crucially needed for fetal brain development, and its supplementation is potentially lowering the risk of neural tube defects and occurrence of autism. Cellular based therapies, specifically with neural progenitor cells, exhibit significant improvements in several neurological conditions including ASD. Multifactorial beneficial therapeutic effects of cell-based therapies with neural progenitor (precursor) cells are achieved via the homing effects with further proliferation and differentiation potential of the stem cells, as well as through multiple paracrine effects – the massive production of cytokines, chemokines, tissue repair-related growth factors, and neurotrophic factors (Figure 5).9,10,100
During the past 20 years, we have offered comprehensive therapeutic solutions to 590 ASD patients from 16 different countries, including 413 boys and 177 girls (Figure 6).
In the entire cohort, a total of 910 cell-based therapy sessions were administered. Among these, 242 patients underwent a single stem cell therapy session, while the remaining 348 patients received multiple infusions of precursor stem cells. As noted in our earlier research, "precursor stem cells are specialized stem cells that are organ-specific and are structurally and functionally aligned with particular regions of the central nervous system.".9. The specifics of the precursor stem cell protocols have been detailed in our previous publications, and revisiting these aspects is not the focus of the current paper.9,10,100 The clinical approaches to design of a specific cellular therapy protocols in each particular case were also discussed in details in previous works. 9, 10,101–103 The age distribution in the observed cohort is given in the Figure below (Figure 7–9).
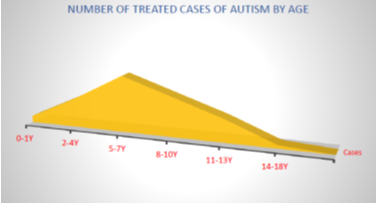
Figure 7 The trend of ASD cases presentation by the families and caretakers for treatment according to the age category in the observed cohort.
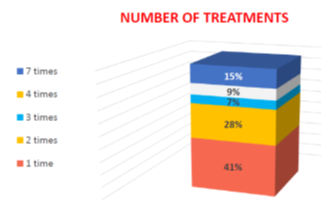
Figure 9 The number of cell therapy sessions with precursor stem cells in the observed cohort of ASD individuals.
The amount of cellular therapies per patient among the 348 patients receiving multiple cell-therapy sessions varied from 2 to 7 implantations (chart). Among the 348 ASD individuals who received multiple stem cell engraftments, 165 (28%) underwent 2 sessions, 41 (7%) had 3 sessions, 53 (9%) completed 4 sessions, and 89 (15%) experienced 7 sessions. The intervals between these sessions varied from 4 to 18 months, based on the severity of ASD symptoms and the patients' clinical responses.
Patients treated with these comprehensive, pathogenetically-based therapeutic protocols generally began to manage their behaviors independently and acted appropriately in various settings, including home, school, family environments, and the community. Positive changes in social and language development were often observed within the first two weeks. By 2-3 months, improvements such as increased eye contact, better attention span, responsiveness to verbal instructions, and the development of meaningful speech were noted. By six months, most children demonstrated greater communication fluency and improved self-management abilities. With ongoing treatment, patients exhibited better management of anxiety, mood disorders, attention deficits, and depression, alongside enhanced social skills. The therapies also helped in managing seizures, gastrointestinal issues, dietary imbalances, and disrupted sleep patterns. The chart below illustrates the changes in ATEC scores before and after treatment. The treatment response rate was effectively 100%, as nearly all individuals in the cohort showed improvements in various developmental areas, including cognitive, speech, or behavioral aspects, if not all of them (Figure 10).
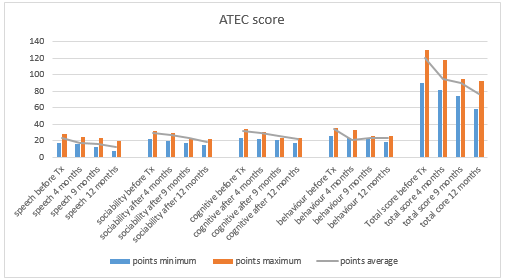
Figure 10 Dynamics of the ATEC score in the treated ASD individuals before the treatment, and after – 4, 9, and 12 months later. Minimum, maximum and mean score in observed ASD cohort (N=590).
To improve the effectiveness of our integrative holistic approach for ASD, we have introduced advanced neuropeptides, growth factors, and tissue repair-related factors derived from fetal neural precursor stem cell cultures. These neuropeptides are crucial for regulating higher brain functions, including both conditioned and unconditioned reflexes, and complex cognitive processes that facilitate appropriate behavior across various social and environmental contexts. Central nervous system peptides play an essential role in managing emotional and social behavior, as well as supporting speech and cognitive development.
The incorporation of mitochondrial peptides and neuropeptides following the engraftment of neuronal precursors has resulted in a marked decrease in anxiety and substantial improvements in behavioral outcomes for individuals with autism. These innovative CNS peptides contribute to increased self-confidence, reduced aggression, and enhanced communication skills and social adaptability. Additionally, the inclusion of extracts from the pineal gland in our treatment regimen addresses sleep disturbances commonly experienced by ASD patients.
It is noteworthy that after 4-6 months of therapy, patients typically show improved conversational abilities, a reduction in repetitive motor behaviors, and a more adaptable approach to routines. Moreover, there are observable advancements in higher cognitive functions, including better peer interactions, increased social and emotional reciprocity, and progress in speech development, verbal communication, and learning.
In addition to the innovative regenerative biological therapies described earlier, children with autism may also benefit from Hyperbaric Oxygen Therapy (HBOT) due to its ability to improve cerebral perfusion. By breathing oxygen at pressures greater than atmospheric levels, HBOT can increase the arterial oxygen partial pressure, thereby enhancing oxygen delivery to the brain. This therapy also exhibits anti-inflammatory properties by decreasing levels of pro-inflammatory cytokines, including tumor necrosis factor-α, interferon-γ, and interleukins 1 and 6. Furthermore, HBOT supports mitochondrial function and boosts the production of antioxidant enzymes.
To further augment the therapeutic effects and address the cortical mechanisms of excitability, connectivity, and plasticity, which are often disrupted in ASD, we also integrate transcranial electric and electromagnetic stimulation therapy. This method uses rapid pulses of electrical current to generate fluctuating magnetic fields, which in turn induce electrical currents within the brain tissue, thereby modulating these critical mechanisms.104 Transcranial stimulation not only affects the targeted cortical areas directly beneath the electrode but also has broader influences on connected brain regions through network interactions. This means that stimulating one cortical area can affect entire neural networks or systems. As a result, clinical applications of transcranial stimulation therapy can lead to significant improvements in behavioral challenges faced by individuals with ASD.
Incorporating regenerative medicine techniques into the treatment of Autism Spectrum Disorders (ASD) marks a significant breakthrough in therapeutic strategies. This analysis reveals that advanced methods, such as cellular therapies with neural progenitor cells, neuropeptide treatments, neuromediator precursors, and nutraceuticals, have shown considerable promise in enhancing behavioral, cognitive, and social functions in individuals with ASD. The observed rapid improvements in self-regulation and behavior, along with lasting gains in communication and social skills, highlight the potential effectiveness of these innovative therapies. Clinical data indicate that a comprehensive approach that combines biological and nutraceutical treatments can markedly improve therapeutic outcomes. These results not only offer hope for more effective ASD management but also emphasize the importance of ongoing research to refine these methods and confirm their long-term benefits. Continued exploration will be essential for optimizing these novel treatments and ensuring they provide sustained, meaningful improvements for individuals with ASD.
None.
The authors declare that there are no conflicts of interest.

©2024 Klokol, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.