Journal of
eISSN: 2373-6410


Research Article Volume 12 Issue 2
1School of Biological Science and Medical Engineering, Beihang University, China
2Beijing Key Laboratory of Rehabilitation Technical Aids for Old-Age Disability, National Research Center for Rehabilitation Technical Aids, China
3The Second Affiliated Hospital of Nanchang University, Nan Chang City, Jiang Xi Province, China
†These authors contributed equally
Correspondence: Xinying Shan, School of Biological Science and Medical Engineering, Beihang University, China
Received: January 11, 2022 | Published: March 23, 2022
Citation: Shan X, Liu S, Wei C, et al. Abnormalities of event-related desynchronization in patients with lower limb amputation. J Neurol Stroke. 2022;12(2):33-38 DOI: 10.15406/jnsk.2022.12.00496
Purpose: Lower limb amputation induces extensive structural and functional reorganization of the brain. Motor imagery has been shown to promote motor recovery and phantom-limb pain alleviation in amputees. However, the neural mechanism of motor imagery in lower-limb amputees is unclear. In this study, we aimed to find electrophysiological evidence of abnormalities from event-related (de)synchronization (ERD/ERS) measurements.
Methods: Twelve patients with lower limb amputation and ten age- and sex-matched healthy controls were recruited. The participants completed motor imagery paradigm experiments while concurrently recording electroencephalogram (EEG). In this study, we chose the C1 and C2 electrode channel. For data analysis, the electrophysiological performance of the time-frequency distribution and event-related desynchronization (ERD) of amputees and healthy controls was assessed and statistically analyzed.
Results: The results showed obvious ERD phenomena in the alpha (7-12 Hz) and low beta (13-20 Hz) frequency bands in healthy controls. When imaging the left leg, there was a significant difference between the healthy and amputees for the alpha in C2 (t =-2.81, p =0.011) and for the low beta band in C2 (t =-2.34, p =0.030). When imagining the right leg, there was a significant difference in the alpha in C1 (t = -2.53, p =0.020) and C2 (t =-2.49, p =0.022), and for the low beta band in C2 (t =-2.58, p =0.018). Furthermore, healthy subjects had a smaller mean ERD in the beta band than those with lower limb amputees.
Conclusions: The difference between lower limb amputees and healthy controls may be related to brain remodeling in amputees. It seems difficult to generate the corresponding ERD/ERS signals, which makes this technology unsuitable for immediate application to amputees.
Keywords: lower limb amputees, reorganization, motor imagery, ERD
What is known: A major amputation is known to seriously affect patient quality of life. The study has shown changes in behavioral patterns in patients that have led to structural and functional reorganizations of the nervous system.
What is New: The lower limb amputee could not be observed ERD phenomenon in task state. amputation changed time-frequency distribution during motor imagery, ERD analysis could be used to detect the deviant functions of cortical activities in LLA, and may provide indices that could be used in clinical researches of LLA.
Human brain plasticity refers to the capacity of the nervous system to reorganize the structures and functions of the brain in response to learning, disease, and experience.1 Individuals suffering from lower limb amputation experience a loss of sensory inputs and motor deprivation, and plasticity occurs at different levels in the central nervous system.2 Accumulated evidence has shown functional reorganization in the primary somatosensory and motor cortical areas in amputees.3 For instance, a previous positron emission tomography (PET) study showed that the local cerebral blood flow of the denervated cortex on the opposite side was significantly increased.4 In addition to the sensorimotor network, brain reorganization has also been observed in other regions following amputation.5 For instance, upper-limb amputees demonstrated anatomical alterations in the dorsal visual cortex.6 It seems that extensive brain reorganization occurred in individuals following amputation.
An electroencephalogram (EEG) is composed of local and nonlocal frequency components. The local component is the natural frequency of a specific cortical area, and the nonlocal component is the frequency that is generated in the non-specific area and can be recorded in most areas of the scalp. These local components are closely related to a special inner neural network state. When a certain cortical area becomes active, the rhythmic activity of a specific frequency is manifested as a decrease in amplitude, which is called event-related desynchronization (ERD); when a certain activity does not make the relevant cortical area significantly active at a certain moment, the specific frequency manifests as an increase in amplitude, which is called event-related synchronization (ERS). The natural frequencies of the motor sensory cortical area are typical local components of EEG, such as the μ(mu), alpha, and beta bands. ERD and ERS have become important methods for studying brain plasticity.
Motor imagery is the internal simulation of sports behavior, but it is not accompanied by obvious physical activity.6,7 Motor imagery has been used for motor rehabilitation of amputees, especially at the early stage of rehabilitation, when amputees cannot cooperate with any form of physical rehabilitation or active sports training.8 Motor imagery can induce activation in a number of brain regions such as the premotor area, auxiliary motor area, parietal cortex, cingulate gyrus, basal ganglia, and cerebellum.9 A brain-controlled prosthesis based on a brain-computer interface has been proposed as an assistive device to improve the living ability of amputee patients. Among them, a prosthesis controlled by motor imagination based on ERD/ERS indicators was used. ERD/ERS experiments were designed specifically for amputees. However, it may be difficult for amputation patients to generate the corresponding ERD/ERS signal due to the degeneration of the sensorimotor cortex, which makes this technology unable to be applied in practice. Lyu and colleagues investigated the relationship between motor imagery and phantom limbs using a hand mental rotation task using behavioral and electrophysiological measures.10 The results showed that ERD-beta was significantly correlated with phantom vividness.
The current study designed a motor imagery experiment for patients with amputations and aimed to investigate the difference between amputees and normal individuals on synchronization/desynchronization signals related to motor imagery-induced events. The findings would provide a criterion for the feasibility of brain-controlled prosthetic research design and provide insights for amputee rehabilitation. This study is the first to show how EEG can be used to evaluate cortical reorganization and activity in the motor cortex of lower limb amputees. Research on cortical reorganization following lower-limb amputation is scarce. The aim was to compare ERD activity during specific movement tasks in healthy and amputated subjects. The objective was to gain further knowledge on the effect of amputation on the motor cortex of the brain.
Twelve patients with lower limb amputation (two females), ranging in age from 32 to 66 years (age:46.58±10.14 years, sex: 10M/2F) were recruited from the Affiliated Hospital of the National Research Center for Rehabilitation Technical Aids, Beijing, China. Amputations were caused by traumatic injury in nine participants and disease in three participants. Five amputations occurred at the transfemoral level and seven at the transtibial level. The duration of having lived with an amputation was 13.75±19.76 months. Ten age- and sex-matched healthy controls (age: 36.55±10.14 years, sex: 9M/1F) were recruited from the local community. All participants grew with a common geographic background (Beijing, China) and had the same ethnic identification ( Han Chinese), while they were people from different socioeconomic and educational backgrounds. Independent sample t-test results indicated no significant difference in age between the two groups. All participants were right-handed, and none of them had a history of neuropsychiatric disorders. This study was approved by the Ethics Committee of the National Research Center of Rehabilitation for Technical Aids. Each participant was informed of the purpose and methods of the study and provided written consent prior to participation in the study.
Experimental paradigms
All subjects were provided with detailed instructions regarding how to perform the task while staying relaxed and reducing unnecessary muscular movement. Before the experiment, subjects were presented with a number of training trials to familiarize themselves with the tasks. For each experimental trial, subjects were instructed to pay attention to the screen of the computer responsible for displaying the stimuli. During the experiment, each subject was seated in a comfortable chair at a distance of 90 cm from a computer screen in a quiet and bright room. Each trial was started with a 2s black fixation cross displayed on the center of the screen. An arrow then appeared at the center of the screen and pointed randomly to one of the two different motor imagery tasks (left lower limb movement, right lower limb movement) for 5s. At the end of each trial, there was a 3s rest. The experiment lasted approximately 25 min, consisting of two blocks, and there were 60 trials in each block. There was a 5-min inter-block break for the participant to rest. During the entire experiment, all subjects were required to avoid any additional facial or arm muscular movements. The subjects were informed that they could terminate the experimental session at any point without questions.
EEG recordings and preprocessing
Continuous EEG was acquired with dual 256-channel Net Station Acquisition EGI dense array EEG using Net Station 4.5 software. EEG electrodes were distributed across the whole head with all electrode impedances below 50 kΩ before recording was started. The recordings were referenced to the Cz electrode. All data were collected at a sampling rate of 500 Hz. Continuous EEG data were preprocessed offline using EEGLAB in MATLAB,11 and we chose a 10-10 system for 70 channels analysis, including the core channels (CZ, C1, C2, C3, C4, C5, C6).The data were digitally filtered between0.1 Hz and 30 Hz. Trials with time-locked movement responses were extracted from the filtered data. The data were divided into left lower limb movements and right lower limb movement conditions. The time period of a single trial was 1000 ms before the response onset to 5000 ms after the response onset, baseline was 1000ms. Bad channels (defined as those with EEG max-min >200 μV after smoothing with a moving average of 80 ms long) were identified and replaced using spherical spline interpolation. Epochs with artifacts due to eye blinks or ocular movements were rejected. Overall, 8.37% of trials were rejected. Artifacts such as blinks, lateral eye movement, voltage drifts, EMG, and ECG were removed from the EEG signals via independent component analysis (using the run ICA Infomax algorithm provided by EEGLAB). The components were removed only when they were consistently marked as artifacts by two researchers. The remaining epochs for each participant were averaged.
EEG analyses
The time –frequency representations (TFRs) of powers were computed using wavelet analysis. This was performed by convolving the complex Morlet wavelets with single-trial EEG data, as described by Cohen. We used wavelets with frequencies ranging from 1 Hz to 30 Hz in steps of 1 Hz. In this study, C1 and C2 electrodes were associated with left lower limb movement and right lower limb movement, and we extracted the average value of the alpha frequency band (7-12 Hz) and the low beta frequency band (13-20 Hz) on the C1 and C2 electrodes to plot the waveform. Power was calculated as the squared norm of the resulting frequency-specific time courses of the complex numbers. Power was averaged across trials and then normalized as change relative to the mean power in a baseline interval from -800 ms to -200ms, using the following formula: The relatively long epochs (−2 s to 5 s) were used for time–frequency decomposition to allow edge artifacts to subside outside the time window of interest (-1 s to 4 s), then data outside of this time window were trimmed off, and the calculation formula is:
Statistical method
Under different channel and motion imaging conditions of left lower limb movement and right lower limb movement, the average value of the ERD/ERS data of 0-4 seconds alpha and beta of each subject was extracted for statistical analysis. In 0-4 seconds, each subject had eight mean values under different conditions, such as the mean value of healthy subjects' movement imagination in the alpha band of the C1 channel. The t-test was performed on the mean values of 0-4 seconds extracted from normal people and lower limb amputees under the same conditions. All statistical analyses were performed using the SPSS version 22. Two-tailed t-tests for independent samples were used to assess the group differences.
Figure 1, Figure 2a & 2b illustrates the time-frequency diagrams of the primary motor cortex (C1, C2) of healthy controls and amputees under motor imagery conditions. Compared with amputees, a significant mu rhythm (7-12 Hz) and low beta frequency (13-20 Hz) after stimulation can be observed in healthy controls.
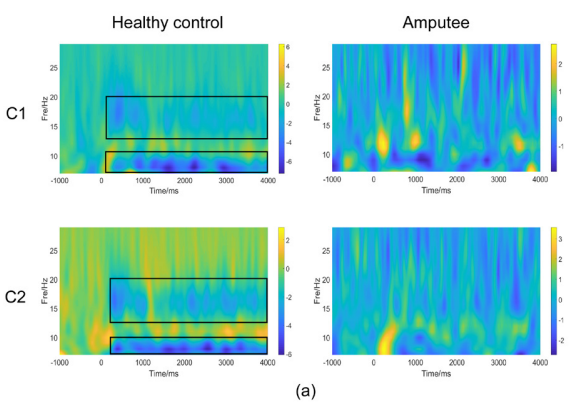

Figure 2 Result of time–frequency analyses in amputees and healthy controls. Figure (S) shows time–frequency representations of subjects performed left leg motor-imagery task. Figure (B) shows time–frequency representations of subjects performed right leg motor-imagery task.
Figure 3 shows the alpha ERD curves of amputees and healthy controls. The ERD curve is based on the data of -0.8 s to -0.2 s in C1 and C2 after the stimulation. Compared to the ratio of baseline, significant ERD phenomenon in the alpha band after the stimulus was presented in healthy subjects, and the curve was negative. However, the lower limb amputees did not see a significant ERD phenomenon in the alpha band after the stimulation, and the curve was positive. A significant period effect was found for alpha band ERD values.
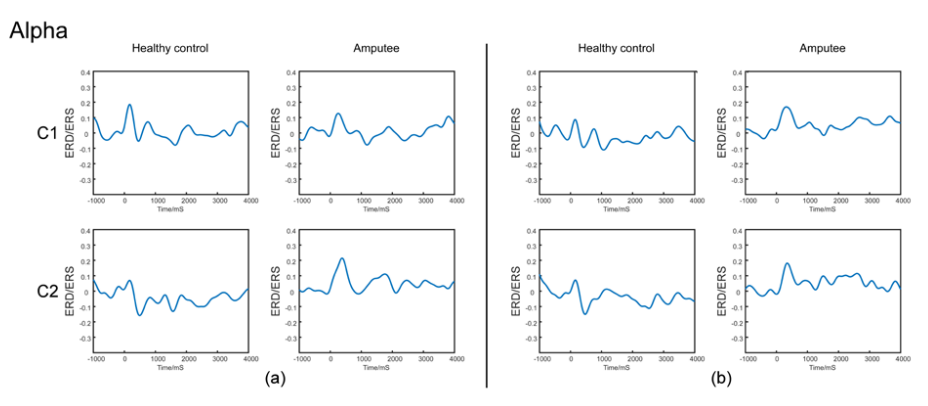
Figure 3 Lists the Alpha band ERD curve of amputees and healthy controls. The ERD curve is based on the data of -1 s-0 s. Figure (A) shows wave form of subjects performed left leg motor-imagery task in Alpha bands. Figure (B) shows wave form of subjects performed right leg motor-imagery task in Alpha bands.
Figure 4 lists the beta ERD curves of healthy subjects and amputees. The calculation method is the same as the alpha band ERD curve, and the result is similar to the alpha band. Healthy subjects can see significant ERD phenomenon in the beta band after the stimulation, and the curve is negative, while the amputees cannot see significant ERD phenomenon after the stimulation, and the curve is positive (Table 1).

Figure 4 Lists the Beta band ERD curve of amputees and healthy controls. The ERD curve is based on the data of -1 s-0 s. Figure (A) shows wave form of subjects performed left leg motor-imagery task in beta bands. Figure (B) shows wave form of subjects performed right leg motor-imagery task in beta bands.
Subjects |
Gender |
Age at scan |
Level of amputation |
side |
Time since amputation (mouth) |
Phantom limb pain(lever) |
1 |
female |
57 |
Transtibial |
right |
8 |
1 |
2 |
male |
46 |
Transfemoral |
left |
4 |
3 |
3 |
male |
41 |
Transtibial |
left |
7 |
1 |
4 |
male |
49 |
Transtibial |
right |
24 |
1 |
5 |
male |
49 |
Transfemoral |
right |
2 |
3 |
6 |
male |
53 |
Transfemoral |
left |
5 |
2 |
7 |
male |
33 |
Transfemoral |
both |
72 |
0 |
8 |
male |
52 |
Transfemoral |
left |
1 |
0 |
9 |
female |
66 |
Transfemoral |
left |
11 |
2 |
10 |
male |
35 |
Transtibial |
right |
5 |
1 |
11 |
male |
46 |
Transtibial |
left |
22 |
2 |
12 |
male |
32 |
Transtibial |
left |
4 |
0 |
Mean |
46.58 |
|
11.64 |
1.33 |
||
SD |
10.14 |
|
7.59 |
1.07 |
||
Table 1 Detailed clinical characteristics of amputees
Table 2 lists the amputees and healthy controls ERD/ERS t-test results. there will be obvious ERD phenomena in the alpha (7-12 Hz) and low beta (13-20 Hz) frequency bands in healthy controls. The results showed obvious ERD phenomena in the alpha (7-12 Hz) and low beta (13-20 Hz) frequency bands in healthy controls. When imaging the left leg, there was a significant difference between the healthy and amputees for the alpha in C2 (t =-2.81, p =0.011) and for the low beta band in C2 (t =-2.34, p =0.030). When imagining the right leg, there was a significant difference in the alpha in C1 (t = -2.53, p =0.020) and C2 (t =-2.49, p =0.022), and for the low beta band in C2 (t =-2.58, p =0.018). Furthermore, healthy subjects had a smaller mean ERD in the beta band than those with lower limb amputees.
Left |
Right |
|||||||
Alpha |
HC |
PT |
T |
P |
HC |
PT |
T |
P |
C1 |
-0.0409 |
0.0134 |
-0.9841 |
0.3368 |
-0.0847 |
0.0673 |
-2.5297 |
0.0199* |
C2 |
-0.0633 |
0.0575 |
-2.8109 |
0.0108* |
-0.0824 |
0.0643 |
-2.4904 |
0.0217* |
Beta |
||||||||
C1 |
-0.0516 |
0.0132 |
-1.2865 |
0.213 |
-0.092 |
0.0094 |
-1.9362 |
0.0671 |
C2 |
-0.1177 |
0.0172 |
-2.3382 |
0.0299* |
-0.1192 |
0.0174 |
-2.5802 |
0.0179* |
Table 2 Statistical result
The table shows the mean power of the healthy control and lower limb amputees in the alpha and beta bands. The table lists the healthy controls and amputees’ ERD/ERS statistical results. The significance level was set at *p < 0.05.
This study aimed to investigate functional changes in brain regions related to motor imagery in left lower limb amputees from the perspective of functional density changes through task-state EEG. The results showed that at the C1 and C2 electrodes, which are related to motor function, there was a significant difference in the beta frequency ERD between the amputation group and the healthy group. When a certain area of the cerebral cortex starts to activate, the metabolism and blood flow of this area increase, and simultaneous information processing can cause the amplitude of the brain wave alpha and beta bands to decrease or block the ERD.12 After the stimulation is presented in normal people, there will be obvious ERD in the alpha rhythm (7-12 Hz) and low beta band (13-20 Hz). PfurtscheUer of the Graz University of Technology in Austria has conducted extensive research on exercise-related tasks.13 He confirmed that the alpha and beta bands were sensitive to movement. In the study of ERD/ERS in the alpha band, when preparing for exercise, the 10 Hz ERD mainly appears in the sensorimotor area on the contralateral side of the scalp, and when the exercise starts, the ERD spreads to the bilateral sensorimotor area, so many researchers speculate that the 10 Hz ERD it is an indicator of brain activation.14 In the study of beta band ERD/ERS, when preparing for exercise, 15 Hz ERD mainly appears in the sensorimotor area on the opposite side of the scalp; when the exercise starts, the ERD expands to the bilateral sensorimotor area.15 When the exercise is over, beta ERD not only returns to the baseline quickly but also shows a short post-exercise ERS.16 Normal people will have obvious ERD in the alpha band and low beta band (13-20 Hz) after motor imagery. Motor imagery can activate related functional brain areas.
These findings are consistent with those of previous studies on neuroplasticity following amputation. Prior studies have reported large-scale changes in neural networks, including and beyond sensorimotor areas following amputation. The cortical representation of various body parts constantly changes based on the pattern of afferent nerve impulses, and if a cortical area is deprived of its input, for example, after an amputation, adjacent cortical areas of the sensory cortex will occupy the silent areas.17 When specific movements of the phantom limb are attempted, the cortical area of the phantom limb may move out of the original area into bordering cortical areas for other body parts.18 Neurophysiological studies have shown that with the development, learning, or pathology of the representative cortex, the cerebral cortex undergoes structural changes and functional reorganization, which is called plasticity.1,19 For example, learning and development can facilitate specific neural circuits and reduce activation, and amputation is also a strong driving factor for brain plasticity.
We did not observe significant ERD phenomena in the alpha and beta bands after the stimulus in the lower limb motor imagery task of the lower limb amputees. After lower limb amputation, the cerebral cortex lacks the corresponding input of the lower limb sensory motor and limb vision, and the maintenance of brain sensorimotor cortex muscle memory depends on the limb sensory motor and visual input.20 When studying the functional connection of the amputation brain, the primary sensory cortex and/or primary motor cortex will detect the expansion of the cortical activation area and the weakening of the functional connections between the cerebral hemispheres.21,22 This phenomenon is interpreted as a maladaptation in the body's recovery process when the body responds to the dysfunction caused by damage to sensory input or conscious control. The lower limb amputees experienced a decrease in vividness during the motor imagination process of the limbs, especially the visual motor imagination scale (KVIQ) score in the lower limb imagination process, was lower than that of the uninjured side.23 The ERD/ERS degeneration of lower limb amputees did not show obvious lateralization in the results. ERD/ERS disappeared in both the left and right brains, which may indicate that degeneration of the left and right motor cortex is synchronous. It is also possible that due to the long-term lack of leg exercises such as walking, the lower limbs are used and retreated for objective reasons. Lower limb amputees do not see significant ERD in the alpha and beta bands similar to normal people after the stimulation is presented. The ERD phenomenon is characterized by structural changes and functional reorganization of the cerebral cortex after amputation. In motor imagery tasks, lower limb amputees did not have the same ERD/ERS phenomenon as healthy people. This problem needs to be considered when designing existing prosthetic assistive devices with brain-computer interfaces.
However, we also studied a case of upper limb amputee and found that the ERD/ERS phenomenon still exists. The degeneration of the leg motor cortex did not affect the motor cortex of the hand. Although the two are close in structure, they are independent of each other in their functional connections. At present, many ERD/ERS control brain-computer interface technologies are used for the motor imagination of the left and right hands of the upper extremity, and few studies have been conducted on the lower extremities. Therefore, this study is of great significance for the study of lower-extremity amputees. In the follow-up, we can collect several cases of upper extremity amputees and perform statistical analysis on the data of upper and lower extremity amputees.
Brain plasticity in amputees is also often associated with prosthetic and phantom limb pain. The use of prostheses prevents a decline in cerebellar gray matter after lower limb amputation. Phantom limb pain is a complication of amputation.24 Many studies have shown that reorganization of the primary body surface sensory cortex may be an important cause of phantom limb pain.25–27 The higher the degree of primary body surface sensory cortex reorganization, the stronger the pain of the phantom limb pain. In cortical reorganization, if certain body surface locations are touched, phantom limb sensation may be induced. These areas are called "trigger zones" Among amputees, both reaction time and beta ERD were positively correlated with the degree of the phantom limb. The central and parietal beta ERD showed angular differences.10 Although the motor imagination of amputees is not lost due to the loss of limbs, their speed is significantly reduced. Phantom limb pain caused by the reorganization of the primary body surface sensory cortex is also an important factor in motor imagery EEG results after amputation.
Motor imagery can activate the plastic potential of the brain’s own cells and repair the functional control connection between the external limbs and the brain. The brain-computer interface based on motor imagery is a type of brain-computer interface generated by motor imagery to realize the human brain and external devices for communication and control.28,29 The main purpose of the current BCI research is to generate EEG signals that are easy to interpret and have obvious characteristics, and then use signal processing algorithms such as feature extraction and pattern classification to identify such EEG signals and make a difference. The same choice or instructions are used to achieve environmental control or exchange of ideas. The biggest advantage of the brain-computer interface system based on motor imagery ERD/ERS is that the EEG signal used for control is only generated by imagination and does not rely on any sensory stimulation.30,31 The experimental design is simple, and asynchronous communication can be achieved. It is the true BCI system, and users can produce it without training or a small amount of training.
Motor imagery can activate the brain in amputees and healthy controls. Alpha (7-12 Hz) ERD and low beta (13-20 Hz) ERD appeared in healthy controls after the stimulation task, but not in amputees. Amputation is a powerful driving factor in brain plasticity. Alpha and beta did not show significant ERD in motor imagery, which is the EEG manifestation of the structural and functional reorganization of the cerebral cortex after amputation. In future studies, we plan to design motor imagery training through a lot of exercises to observe whether patients with amputation can recover the ERD/ERS phenomenon. This can explain whether the plastic degradation of the primary motor cortex of amputation patients is reversible, and whether the brain-computer interface based on motor imagination can improve the reliability of amputation patients through training.
Xinying Shan conceived and designed the experiments; Xinying Shan and Shaowen Liu analyzed the data and performed the experiments; Yan Zhang contributed reagents and materials; Shaowen Liu and Conghui wei wrote the paper.
The present research was approved by the Ethics Committee of laboratory animals of Nation Research Center of Rehabilitation for Technical Aids. we obtained with the informed consent of all participants.
Thank all participants for cooperating with our experiment.
This research was supported by National Key R&D Program of China (2020YFC2005801).
The authors declare no conflict of interest.

©2022 Shan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.