Journal of
eISSN: 2373-6410


Case Report Volume 11 Issue 4
Division of Pediatric Neurology, Pediatric Department, King Khalid University Hospital, College of Medicine, King Saud University, Riyadh, Saudi Arabia
Correspondence: Dr. Amal Y Kentab, Department of Pediatrics (39), King Khalid University Hospital, College of Medicine, King Saud University, P.O. Box 2925, Riyadh 11461, Saudi Arabia
Received: July 02, 2021 | Published: August 16, 2021
Citation: Kentab AY. A rare case of bilateral optic neuritis and acute disseminated encephalomyelitis post mycoplasma pneumoniae infection. J Neurol Stroke. 2021;11(4):122-125. DOI: 10.15406/jnsk.2021.11.00469
A case of a 4-year-old boy who developed acute disseminated encephalomyelitis (ADEM) and optic neuritis (ON) following Mycoplasma pneumoniae infection is reported. His symptoms, including excessive sleepiness, frontal headache, bilateral vision impairment, retro-auricular pain and unbalanced gait, were resolved after methylprednisolone pulse therapy, intravenous immunoglobulin (IVIG) and ciprofloxacin. Cerebrospinal fluid myelin basic protein (MBP) and Mycoplasma serology IgM were detected in our patient. This is the first report of a child with ADEM and ON associated with mastoditis caused by M. pneumoniae infection. Combined immunomodulatory therapy (pulse steroids and immunoglobulin) with anti- mycoplasma microbial therapy resulted in favorable visual recovery. Bilateral isolated optic neuritis as the first presenting feature in childhood ADEM is rare and requires proper evaluation and early therapeutic management. This case highlights the need for physician awareness of the association of mycoplasma infection with optic neuritis and ADEM.
Keywords: acute disseminated encephalomyelitis (ADEM), optic neuritis (ON), mycoplasma pneumonia, children
Mycoplasma pneumoniae (M. pneumoniae ) is an important cause of upper respiratory tract infections and community acquired pneumonia. Extrapulmonary neurological complication include immune mediated disorder such as optic neuritis, acute disseminated encephalomyelitis, Guillain–Barre syndrome, and transverse myelitis.1–3 Neurological manifestations have been reported to occur in 0.1% - 7% of patients infected with M. pneumoniae infections typically occurring between 2 and 14 days after initial respiratory symptoms.2
Optic neuritis (ON) or inflammation occurs either in isolation (idiopathic), associated with bacterial, viral, or parainfectious etiology or in the context of a more widespread demyelinating disease such as ADEM, multiple sclerosis (MS) or isolated clinical syndrome (CIS). In children, the tendency of having bilateral visual loss which is secondary to anterior optic nerve involvement with papillitis is high. Visual recovery is better in children than adults. It is less often associated with MS in children than it is in adults. Note worthily, approximately one third of affected children may develop MS in the future.4,5
Acute disseminated Encephalitis (ADEM ) is a well-known autoimmune –mediated monophasic inflammatory demyelinating disease of the central nervous system that principally involves the white matter of the brain and the spinal cord, although the grey matter (basal ganglia, and thalami) may also be affected and is often preceded by a viral infection or vaccination in 50-70% children or young adults.6,7 It has been reported also in association with other non-viral infections as mycoplasma pneumoniae.8 Patients often demonstrate diversity of clinical features which include altered sensorium, convulsion, paresis, and other signs of CNS involvement.9,10 Bilateral optic neuritis as the initial presenting feature of ADEM is very rare and reported only in 2% of these cases.11 It is a diagnosis of exclusion and relies on neuroimaging which may be normal at onset. This is the first report of a child developing ADEM and ON following mastoditis that is caused by M. pneumoniae infection.
Patient`s information: A 4-year-old boy presented with sudden onset of diminution of vision in both eyes with mild pain on ocular movement of 3 days duration. There was a history of frontal headache with pain behind the ears on the right more than the left. There was no previous history of any chronic medical disorders or recent vaccinations. There was no history of altered sensorium, convulsion, weakness or fever. He had a history of viral – like illness 10 days prior to presentation.
Clinical findings
On examination he was conscious with normal mental status. He had sluggish pupillary reaction (afferent pupillary defect) bilaterally with visual acuity of just perception of light and accurate projection of rays. Fundus examination revealed bilateral disc hyperaemia with oedema, with no peripapillary flam – shaped haemorrages which was suggestive of optic neuritis. A slit-lamp examination of the anterior segment was also normal in both eyes. Because of the patient's very poor vision, visual field testing and color vision testing were precluded. He had a normal extraocular movements with no nystagmus, or facial weakness. The cranial nerve examination was normal. Tone, muscle strength, and deep tendon reflexes were normal in extremities, although mild hyperreflexia with no clonus was bilaterally noted in lower limbs. Plantar responses were equivocal bilaterally. Cerebellar signs were absent. He was able to walk with support but with ataxic gait. Systemic examination was unremarkable apart from mild tenderness behind the right ear with bilateral otitis media.
Diagnostic assessment
Basic laboratory work-up revealed raised erythrocyte sedimentation rate 30 mm/hr with negative C reactive protein, and normal complete blood count. Blood chemistry, renal and liver profiles were normal. Vitamin D level was low 48 nmol/l (NR 75- 120). Vasculitis work-up including antinuclear antibody (ANA) test, anti-DNA antibody test was negative. Nasopharyngeal aspirate for viruses, blood culture, cerebrospinal fluid (CSF ) analysis, cultures and PCR for viral multiplex, throat swab, antistreptolysin (ASO) titer, viral serology ( EBV, CMV, herpes simplex type I, varicella ) were unrevealing, while screen for mycoplasma pneumonia showed positive IgM . Respiratory and CSF PCR for M. pneumoniae were not done. CSF myelin basic protein (MBP) was positive while oligoclonal bands were negative. Plain brain computed tomography (CT scan) was normal, but showed bilateral opacification of mastoid air cells more at the left side consistent with bilateral mastoiditis (Figure 1). Orbital and brain magnetic resonance imaging (MRI) showed diffuse and symmetrical thickening of both optic nerves with enhancement (Figure 2), and intracranial demyelinating features with multiple lesions at grey-white matter junction, and cerebellar peduncle with and non – specific white matter enhancement (Figure 3). Visual evoked potential (VEP) showed a prolonged latency suggestive of optic neuritis.
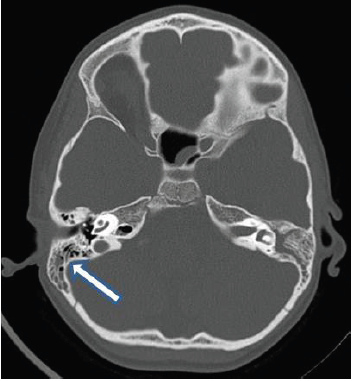
Figure 1 Computed tomography (CT) (Plain) shows bilateral opacification of mastoid air cells suggestive of mastoiditis.
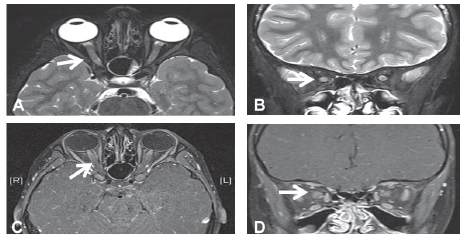
Figure 2 Orbital Magnetic resonance imaging (MRI) (A) Axial T2WI, and (B) Coronal T2WI showing bilateral thickening and protrusion of optic nerves with increased T2 signal intensity. (C ) Axial T1WI post contrast, and (D) Coronal T1WI post contrast showing abnormal enhancement more prominent at the intraorbital segment representing bilateral optic neuritis.
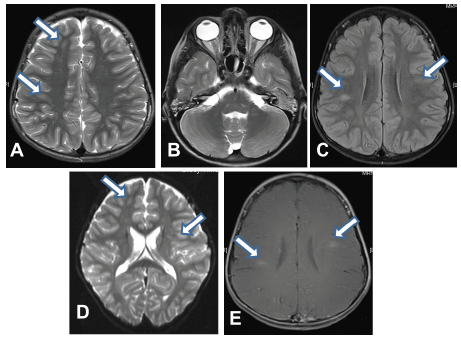
Figure 3 Magnetic resonance imaging (MRI) (A) and (B) Axial T2WI, Showing lesions with high signal intensity at right frontal, right parietal, left middle cerebellar peduncle lesion, respectively. (C) Axial Fluid attenuation recovery image (FLAIR) showing right parietal lesion with hyperintensity, (D) Axial diffusion Image showing right frontal and left frontal lesions with diffusion restriction , and (E) Axial T1WI with contrast showing abnormal enhancement of the right parietal and left frontal lesion with no involvement of basal ganglia, thalami, or corpus callosum.
Therapeutic intervention
The patient was started on intravenous methylprednisolone 25mg/kg/day for 3 days and intravenous immunoglobulin (IVIG) 2g/kg for two consecutive days, followed by oral prednisolone with tapering doses over 6 weeks. Regarding his infection (mastoiditis), he was started on ciprofloxacin for 2 weeks, which is also an effective treatment against mycoplasma pneumonia. He was given vitamin D supplement. He showed initial improvement and appreciated hand movements 72 hours after immunosuppressive therapy.
Follow-up and outcomes
At the three weeks follow-up visit, he exhibited marked improvement with complete resolution of the headaches, and almost normal visual acuity 20/30 in both eyes with mild persisting optic disc edema with normal visual field test, and color vision test on ophthalmological evaluation. He was planned for follow- up MRI to document the radiological recovery, but was lost to follow-up in our center. A follow-up MRI in another center showed almost normal thickness, signal intensity for both optic nerves with no evidence of demyelination.
Our patient developed ADEM and ON following M. pneumoniae infection which is a rare condition.12 Aydin A, et al, 2004 reported ADEM with bilateral transient amaurosis as a complication to Mycoplasma in a 4 –year-old boy that, responded well to IVIG. Optic neuritis (ON) in younger children like our patient is more likely to occur following infection or vaccination, or as part of ADEM.9,10 Antecedent viral infections have been reported in up to two-thirds of children with postinfectious ON . Non-viral causes like M. pneumoniae Infections have been reported in association with ON in children.13–18
Rapid onset of vision loss may suggest a demyelinating disease (ADEM vs MS), and bilateral involvement favor ADEM, and found in a quarter of childhood cases of ADEM.
ADEM is another cause for ON. It`s exact pathogenesis is not properly confirmed, but, however it was suggested to involve antivirial antibodies or a cell – mediated response to the pathogen cross-react with the myelin autoantigens (molecular mimicry). It is characterized by prodromal symptoms of fever, malaise, myalgia, headache, or nausea and vomiting in prepubertal age in 80% of cases, followed by encephalopathic features including restlessness, lethargy, hallucination, confusion and altered sensorium associated occasionally with seizures or vision loss, usually after an interval of 4–21 days.9,10 In addition, focal or multifocal neurological signs such as meningeal signs, hemiparesis, paraparesis, cranial nerve palsies can also manifest.10 Our patient had excessive sleepiness which may reflect encephalopathic picture.
Diagnosis of ADEM depends on MRI findings that include T2 and fluid attenuated inversion recovery (FLAIR) hyperintense multifocal, ill-defined asymmetric, large (greater than 1-2cm) white matter lesions of the cerebrum, cerebellum, brainstem, and spinal cord, which are frequently large.9 Periventricular and T1 hypointense lesions are less common than MS.9 Orbital MRI may reveal ON thickening and enhancement that represent an optic neuritis.7
The following findings support ADEM vs MS in our patient; a) younger age group (<10 years), b) viral - like infection prior to presentation and mastoiditis secondary to mycoplasma at time of presentation, c)presence of bilateral ON, d) typical neuroradiologic findings, e) absence of oligoclonal bands, f) monophasic course of the disease. The presence of MBP supports an ongoing demyelinating disease, although its high level is usually in
favor of MS.
The extrapulmonary neurological complication of M. pneumoniae has been widely reported in pediatric age group.1–3 with encephalitis been the most frequent one. Almost 20% of patients with CNS findings have no preceding or concomitant respiratory infection.2,3
There are few reports worldwide in the English literature of ADEM due to M pneumoniae infection;19 including a report in a young adult from UAE.20 Al-Zaidy Sa, et al.21 reported ADEM in 12% of those patients whom had neurologic disease attributable to M pneumoniae infection.21 The underlying mechanism for the CNS complications include either direct invasion into the CSF or a systemic immune-mediated response provoked by molecular mimicry 2 to 3 weeks after the respiratory disease subsided.21
Diagnosis of M. pneumoniae infection depends on the presence of a consistent clinical picture that coincides with positive serological testing (IgM and IgG titers) such as, enzyme-linked immunosorbent assay (ELISA), and indirect immunofluorescence. Serological tests has its own limitation as they depend on convalescent sera for confirmation, whilst false-positive results from cross-reactivity becomes a problem.1,2 In addition, IgM antibodies vary with age; and usually become positive in acute infection but also may be negative in the course of acute infection or it`s positivity may last for months.22 Seroconversion is defined as a 4-fold increase in titre between acute and convalescent sera, or a single high anti-M. pneumoniae complement fixation antibody titre of >1:128.1 Cold agglutinins had been used in the past as they are produced 1–2 weeks after infection in 50% of patients and may persist for several weeks but they have poor sensitivity and specificity. Microbial culture is seldom used in routine medical practice. The criteria for diagnosis of M. pneumoniae infection that cause CNS complication included detection of M. pneumoniae by culture or PCR in respiratory or CSF samples and/or positive serological testing.1
Standard therapy for the management of ADEM due to M. pneumoniae is not available due to lack of controlled clinical trials and recommendations. Based on multiple case reports immune-modulating therapy, with intravenous methylprednisolone (20–30 mg/kg/day, maximum 1gm/day) for 3–5 days, followed by tapered oral corticosteroids over 4–6 weeks; has a beneficial effect.23,24
The role of antimicrobials treatment remains controversial as it depends on associated mechanism. It is indicted in direct invasion, while if immune – mediated mechanism is suspected, it is not quite understood if antimicrobial therapy is appropriate, particularly after the acute illness has been resolved.1,13 Practically, it is administered in association with steroid when other causative agents have been excluded and should be continued irrespective of prodromal or neurological manifestations until more evidence becomes available.24 The use of others; IVIG at 2 g/kg divided over 2–5 days or plasmapheresis depends on complexity of the case and the rate of responses to steroid therapy.7 Our patient responded dramatically to intravenous steroid therapy and IVIG, and the vision improves over 3 weeks. To the best of our knowledge, this is the first report of a child developing ADEM and ON following mastoditis caused by M. pneumoniae infection.
Although it is rare, M. pneumoniae infections can cause ADEM and bilateral ON, and it should be considered in children presenting with combined picture of encephalopathy and progressive vision loss. Early recognition and promote treatment with immune modulatory, and antimicrobial therapy can save the affected child from varying degrees of life-threatening complications resulting in chronic debilitating deficits in motor or mental or visual function.
Written informed consent for the publication of this case report was obtained from the parents of the patients. The authors declare that ethics approval was not required for this case report.
This work was supported by the College of Medicine Research Center, Deanship of Scientific Research, King Saud University. Also, we are grateful to the parents and patient reported in this article for their genuine support.
The authors declare no Conflicts of interest.

©2021 Kentab. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.