Journal of
eISSN: 2373-6410


Cerebral cortical biopsies of 16 patients with clinical diagnosis of congenital malformations, Arnold- Chiari malformation and postmeningitis hydrocephalus were studied to establish mitochondrial morphopathological alterations. Cortical biopsies obtained in the surgical room were immediately processed by conventional technique for transmission electron microscopy.Three injured mitochondrial morphological patterns were found: Swollen clear mitochondria, swollen dense mitochondria, and dark degenerated mitochondria were frequently observed in hydrocephalic parenchyma. Swollen clear mitochondria exhibited low electron dense matrix, enlarged intracristal space and continuity of outer and inner mitochondrial membranes. Swollen dense mitochondria showed high electron dense matrix and swollen fragmented cristae. Dense degenerated mitochondria displayed overall high electron density of matrix and mitochondrial membranes.Such injured mitochondrial pattern are mainly related with nerve cell death and considered markers of lethal nerve cell injury.
Keywords: mitochondria, congenital hydrocephalus, hydrocephalic edema, electron microscopy
Vuia1 reported two twin brothers diagnosed with congenital spongy degeneration of the brain featured by accumulation of lamellar bodies within the mitochondria, and free in the cellular cytoplasm with tendency to form inclusions of the multilamellar or finger-print type. Watanabe2 described in a 51/2-year-old boy with congenital myopathy associated with communicating hydrocephalus myofibrillar disorganization and Z-band streaming with decrease or absence of mitochondria. These Authors considered that common pathogenetic mechanism may have been involved in the development of both the myopathy and the hydrocephalus. Kaur and Ling3 found swelling and disintegration of mitochondria in ricin-induced hydrocephalus in postnatal rats. Madhavi and Jacob4 carried out a morphometry study of mitochondria in the choroidal ependyma of hydrocephalic guinea pigs, and observed that the inner membrane of the mitochondria including cristae exhibited a significant decrease in the hydrocephalic animals. According to these Authors, this reduction in the surface area could probably be attributed to the reduced activity of choroid ependymal cells in obstructive hydrocephalus. Castejón5 distinguished variable degrees of mitochondrial swelling in human hydrocephalic cerebral cortex. Boillat et al.6 described distorted mitocondria in the deep cortical pyramidal cells of infant rats with inherited hydrocephalus. Castejón et al.7 examined in details the pathological changes of mitochondria in the edematous human cerebral cortex associated to severe and complicated brain trauma, tumors, and in a preliminary way the human hydrocephalus.
Castro-Gago et al.8 carried out an analysis of muscle biopsies and described light microscopic morphology and ultrastructural alterations typical of mitochondrial disorders, and low levels of complexes III and IV of the mitochondrial respiratory chain in congenital hydranencephalic-hydrocephalic syndrome. Morava et al.9 reported mitochondrial dysfunction in Brooks-Wisniewski-Brown syndrome with a significantly compromised of mitochondrial oxidative phosphorylation. These findings raised the possibility that at least some cases of congenital hydranencephalic-hydrocephalic syndrome may be due to alterations in the mitochondrial respiratory chain. Balaratnasingam et al.10 demonstrated mitochondrial cytochrome c oxidase expression in the central nervous system at elevated sites of pressure gradient elevation in hydrocephalus, and emphasized the importance of pressure gradients in regulating mitochondrial function in the central nervous system.
Fine structural changes of mitochondria in experimental cerebral edema have been widely reported since the advent of transmission electron microscopy.11-13 Ultrastructural pathology of neuronal mitochondria, after transient and permanent ischemia and anoxia in experimental animals, have reported that mitochondria undergo a sequence of profound alterations in structure and function, which appear to contribute to cell death.14-18
More recently, Baloyannis19 reported substantial morphological and morphometric changes of the mitochondria in the neurons of the hippocampus, the neocortex, the cerebellar cortex, the thalamus, the globus pallidus, the red nucleus, the locus coeruleus, and the cerebellar climbing fibers.
In the present study we systematically examine the mitochondrial alterations in hydrocephalic neurons, glial cells, and capillary wall. This study has been carry out using cortical biopsies of living hydrocephalic patients obtained at the surgical intervention, as a future step toward characterizing mitochondria as lethal markers of nerve cell death. To the best of our knowledge this approach has not been previously reported thus far.
Cortical biopsies of 16 neonate, infant and young patients, ranging from 10 days to 21 years-old, with clinical diagnosis of congenital hydrocephalus, Arnold-Chiari malformation, postmeningitis hydrocephalus and pinealome of third ventricle were examined with the transmission electron microscope. The Table No.1 contains the clinical data and lists the cortical regions from which the cortical biopsy was taken during neurosurgical treatment. The neurosurgical study was performed and the cortical biopsies were taken according to basic principles of Helsinki Declaration. Two to five mm thick cortical biopsies were immediately fixed in the surgical room in 4% glutaraldehyde-0.1M phosphate or cacodylate buffer, pH 7.4, at 4°C. After 2 hours glutaralhehyde-fixation period, the cortical biopsies were divided into approximately 1 mm fragments and observed under a stereoscopic microscope to check the quality of fixation of the sample, glutaraldehyde diffusion rate and the brownish coloration of the surface and deeper cortical regions, indicative of good glutaralhehyde fixation by immersion technique. The cortical slabs were also performed to assure optimal diffusion rate of secondary osmium tetroxide fixatives. Immersion in fresh glutaraldehyde solution of 1mm slices was done for 2hours. Secondary fixation in 1% osmium tetroxide-0.1M phosphate buffer, pH 7.4, was carried out for 1-2hours at 4°C. Black staining of the cortical slices was also observed under a stereoscopic microscope to check osmium tetroxide diffusion rate and quality of secondary fixation. They were then rinsed for 5 to 10 minutes in phosphate or cacodylate buffer of similar composition to that used in the fixative solution, dehydrated in increasing concentrations of ethanol, and embedded in Araldite or Epon. For proper orientation during the electron microscope study and observation of cortical layers, approximately 0.1 to 1mm thick sections were stained with toluidine blue and examined with a Zeiss photomicroscope. Ultrathin sections, obtained with Porter-Blum and LKB ultramicrotomes were stained with uranyl acetate and lead citrate and observed in a JEOL 100B transmission electron microscope (TEM) at magnifications ranging from 30,000 to 60,000X. For each case, approximately 50 electron micrographs were studied. The material and method has also been published elsewhere.5,7
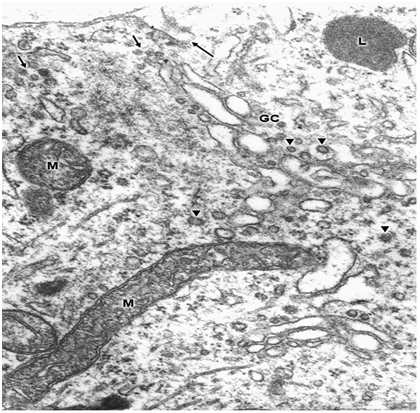
Swollen clear mitochondria were observed in non-pyramidal neurons in all cases under study characterized by swollen clear mitochondrial matrix (Figure 1) (Figure 2). The swollen dense mitochondria appeared as ovoid or enlarged profile organelles with edematous cristae (Figure 3). Both mitochondrial populations exhibited continuous inner and outer mitochondrial membranes, dilated intracristae space, and fragmentation or dissolution of mitochondrial cristae in severe brain edema. Some hydrocephalic neurons showed very long and dark mitochondria (Figure 4) surrounding the Golgi complex region (GC), and apparently associated with large Golgi vacuole. At the level of swollen and degenerated axosomatic presynaptic endings dark mitochondria were found coexisting with clear swollen mitochondria in the notably edematous postsynaptic neuron (Figure 5). In areas of severe hydrocephalic edema we found dark degenerated mitochondria (Figure 6). At the level of capillary wall, the swollen perivascular end-feet astrocytes exhibited dense mitochondria with dilated and fragmented cristae (Figure 7).






In the present study we have found three different types of mitochondrial morphology in neurons, astrocyte cells, and at the capillary wall of patients with congenital malformations and peritumoral hydrocephalus. Solenski et al.19 reported dense cortical neuronal mitochondria exposed to severe ischemic-reperfusion conditions, whereas increasing loss of mitochondrial density with pronounced swelling were observed in permanent ischemia. The present study shows that swollen dense mitochondria appear more frequently at the level of parenchymal regions with sustained and permanent nerve cell ischemia. In addition, we have found in severe hydrocephalic edema enlargement of intracristal space and fragmentation of cristae. The above mentioned findings indicate that mitochondria show significantly different degrees of ischemic injury, expressed by the electron density changes of mitochondrial matrix. The matrix density is apparently related with aggregation of proteins responsible by its omiophilic property. The fragmentation of mitochondrial cristae suggest that oxidative phosphorylation of ADP, the precursors of high-energy phosphate bond of ATP, no longer occurs. In addition, suppose an interruption of mitochondrial membrane intracellular transport, which cause respiration-dependent extrusion of H+ and accumulation of Ca2+ from the cytoplasm.20 During ischemia the lack of oxygen blocks oxidative metabolism so there is no energy to maintain the membrane potential required to drive Ca2+ uptake into the mitochondria.21 However, in brain edema mitochondria can accumulate excessive amounts of Ca2+ and become overloaded.22 When this occurs, mitochondria undergo a permeability transition of the inner mitochondrial membrane,22 release Ca2+, undergo swelling and become uncoupled, thereby losing the ability to produce ATP, .and inducing nerve cell death. Therefore, neuronal death by necrosis or apoptosis depends of mitochondrial function.23 Presumably hydrocephalic or interstitial edema induces a high conductance of mitochondrial permeability transition pore or megachannel24 in the inner mitochondrial membrane, which causes mitochondrial swelling. In this context mitochondrial swelling should be considered as a epiphenomenon25 preceding nerve cell death.
Mitochondria accumulate much of the post-ischemic calcium entering the neurons via the chronically activated N-methyl-D-aspartate receptors contributing to excitotoxicity.26 Calcium overload of nerve cell mitochondria plays a key role in excitotoxic and ischemic brain injury. According to Kristian et al.,23 calcium induces large-amplitude swelling and matrix vacuolization. Calcium accumulation was earlier reported by Gutierrez Diaz et al.16 in nerve cell mitochondria, at the level of the synaptic vesicles, Golgi apparatus, lysosomes and glial and neuronal nuclei. In addition, mitochondria Ca2+ overload excessively activates phospholipases and other degradative enzymes leading to mitochondrial damage.7 Nitric oxide and its derivative peroxinitrite inhibit mitochondrial respiration (complexes I, II and V). NO-induced inhibition of respiration in brain nerve terminals result in rapid glutamate release, which might also contribute to neurotoxicity. Peroxinitrite causes opening of the mitochondrial permeability transition pore, resulting in release of cytochrome c, which might then trigger apoptosis.27
Mitochondrial electron transport also generates reactive oxygen intermediates (ROI). A large increase of ROI induce collapse of mitochondrial membrane potential and neuronal cell death The elevated intracellular Ca2+ and exposure to fatty acids, which alter the physical properties of mitochondrial membranes and inhibition of mitochondrial respiratory components, may enhance this leak of ROI from mitochondria.28,29 Lipid peroxidation occurs also in brain edema following ischemia and hypoxia30 which could be responsible for the mitochondrial membrane damage.
Clinical applications and research on the role of mitochondria in the genesis of human bydrocephalic edema.
According to the neurosurgical study (Table 1), the patients exhibited neurologic symptoms and sequelae, primarily related with the impaired mitochondrial respiration. Impairment of cerebral energy metabolism would explain the functional deficits, neuropsychiatric sequelae, convulsions and disturbance in the state of consciousness in the patient under study. According to our study, mitochondria should be considered as important lethal marker organelle31 which undergo profound changes following ischemia and anoxia. This view is partially in agreement with Wagner et al.32 observations on delayed onset of neurologic deterioration in cats following ischemia/anoxia.
The findings herein described suggest an early and urgent clinical and surgical intervention in child with congenital hydrocephalus in order to avoid mitochondrial damage, nerve cell death and to improve clinical outcome. The many processes involved in injury conditions, such like that cited above, such as free radical formation, intraneuronal calcium influx and calcium-mediated intracellular reactions, excitotoxicity by glutamate and possibly other neurotransmitters, glial oxygen changes, lipid peroxidation and programmed cell death or necrosis, should be considered in conducting a treatment protocol.
Sample identification |
Age and sex |
Clinical data |
Diagnosis |
Biopsy region |
1.HLCS |
1m, F |
Increase of cephalic circumference. |
Uncompensatecommunicant hydrocephalus. |
Right parietal cortex |
2.EEAT |
1m, F |
Increase of cephalic circumference. |
Uncompensate congenital hydrocephalus. |
Right temporo-parietal cortex. |
3LMBC |
1m,F |
Increased cephalic circumference. Hypertensive fontanelles. |
Congenital communicant hydrocephalus. |
Right frontal cortex. |
4. DEMI |
6m,M |
Increased cephalic circumference. Left peridural abscess. |
Congenital hydrocephalus. |
Right temporo- parietal cortex. |
5.FJRA |
10d,M |
Bulding of fontanelles and increased cephalic circumference after surgical correction of lumbar meningomye-locele. |
Arnold-Chiari syndrome. Communicant hydrocephalus |
Frontal cortex. |
6.CMV |
2m,F |
Increased cranial volu-me, hypertensive fon-tanelles, deviation of gaze to the right, external rotations of both legs and increased tendinous re-flexes after treatment of meningomyelo-cele |
Arnold-Chiari malformation. Hydrocephalus.Parieto-occipital abscess. |
Right parietal cortex. |
7.UN |
12d,F |
Increased cephalic circumference after treat-ment of lumbar me-ningomyelocele. |
Congenital hydrocephalus. Meningocele. |
Right parietal cortex. |
8.IATF |
3m,M |
Febril syndrome. Meningitis. |
Postmeningitis hydrocephalus. |
Right frontal cortex. |
9.NSM |
8m,F |
Increased cranial volume since three months. |
Communicant hydrocephalus. |
Right parietal cortex. |
10.GAAPG |
3m,M |
Meningeal syndrome. Tonic-clonic convulsions, increased cephalic circumference. |
Postmeningitis hydrocephalus. |
Frontal cortex. |
11.RGG |
4m,F |
Increased cephalic circumference, hypertensive fontanelles. |
Congenital hydrocephalus. |
Right frontal cerebral cortex. |
12.HR |
2y, F |
Increased cranial volume since 4 months of age. |
Communicant hydrocephalus. |
Right frontal cortex. |
13.ISS |
7m,M |
Increased cranial volume. Diagnosis of subarachnoid hemorrhage after axial computer tomography. |
Communicant hydrocephalus. |
Right frontal cortex. |
14.JM |
21y,M |
Sudden increase of cephalic circumference. Tonic convulsions. |
Pinealome of third ventricle. |
Posterior parietal cortex. |
15.CC |
10y,M |
Increased cranial volume. |
Comunicant hydrocephalus. |
Parietal cortex. |
16.EV |
5y,F |
Increased cephalic circumference. |
Comunicant hydrocephalus. |
Parietal cerebral cortex. |
Table 1 Neurosurgical study.
Three injured mitochondrial morphological patterns were found in hydrocephalic brain parenchyma: Swollen clear mitochondria, swollen dense mitochondria, and dark degenerated mitochondria. Swollen clear mitochondria exhibited low electron dense matrix, enlarged intracristal space and continuity of outer and inner mitochondrial membranes. Swollen dense mitochondria showed high electron dense matrix and swollen fragmented cristae. Dense degenerated mitochondria displayed overall high electron density of matrix and mitochondrial membranes.Such injured mitochondrial pattern are mainly related with nerve cell death and considered markers of lethal nerve cell injury.
No conflict of interest. This research received no specific grant from any funding agency in the public, commercial, or not-for profit sectors.
This study has been partially carried out through a subvention obtained from CONDES-LUZ, Castejón Foundation and San Rafael Home Clinic. Maracaibo. Venezuela.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.