Journal of
eISSN: 2373-6410


Osteogenesis imperfecta (OI) is a genetic disease characterized by reduced bone mass and increased risk of fractures. Among extra skeletal abnormalities, it presents coagulation disorders (vascular fragility, decreased platelet retention, decreased levels of factor VIII and deficient platelet aggregation induced by collagen), mainly hemorrhagic in varying degrees of manifestation We present a 6-years-old child with type 3 OI which after a trivial fall, developed extensive neurosurgical epidural hematoma with posterior complicating ischemia in the middle and posterior cerebral artery territories. Case reports of cerebral ischemia complications in brain hemorrhage after minor, trivial head traumas in normal children have been published, but none associated with OI patients since supposed they use present post traumatic hemorrhagic complications.
Keywords:Osteogenesis Imperfect, cranial epidural hematoma, Brain ischemia, Hematological pathologies
OI, Osteogenesis Imperfecta; GCS, Glasgow Come Scale; CT, Computed Tomography; EDH, Epidural Hematoma; GOS, Glasgow Outcome Scale
Osteogenesis imperfecta (OI) is a rare heterogeneous group of inherited diseases, with an estimated incidence between 1:15.000 to 1:20.000 individuals, characterized by an autosomal dominant mutation, and clinically by low bone mass and increased bone fragility, extra skeletal,1 and hematological abnormalities.2,3
We report the case of a child with type 3 OI and massive epidural hematoma due to trivial fall that developed ischemia in the middle and posterior cerebral artery territories after neurosurgical drainage. We discuss possible associated factors with this complication.
Female patient, 06 years old, with type 3 OI diagnosed since 2 years old, regular use of calcium, falled from an inert hammock about 40 centimeters from the ground with trivial parietal trauma. Four hours after she was admitted in the emergency room in coma, with Glasgow Coma Scale (GCS) 6, anisocoric pupils by left midriasis. Submitted to head Computed Tomography (CT) scan which demonstrated left temporal skull fracture and in the same side a frontotemporoparietal epidural hematoma (EDH) with a midline deviation of 15 millimeters and subfalcine herniation (Figure 1). There were no signs of spinal fractures in radiological emergency screening (Figure 2). Preoperative laboratory tests without significant changes were performed (hemoglobin 10.6 mg/dL; hematocrit 31.8 mg/dL; prothrombin activity 70%; platelets 145.000/mm³, activated partial thromboplastin time 30''; INR 1,6). She was submitted to urgency craniotomy and hematoma evacuation, without operatory hemodynamic or hemorrhagic complications. In postoperative pediatric intensive care, the neurological examination day after after surgery showed a patient with GCS 6, persistent anisocoric pupils by left midriasis. The 24 hours postoperative laboratory tests showed a relative decreased platelet count (117.000/mm³), prothrombin activity (49%) and INR (1,58) without hemorrhagic repercussions. It was performed, 48 hours after surgery, control brain CT that showed signs of ischemia in the adjacent left temporal cortex to evacuated hematoma (Middle Cerebral Artery territory) and left thalamus, midbrain peduncle, occipitotemporal and bilateral calcarine fissure cortex hypodensities corresponding to outlying ischemia in Posterior Cerebral Artery territory without signs of brain hemorrhage or intracranial hypertension (Figure 3). After two months of hospitalization, she was discharged, with a Glasgow Outcome Scale (GOS) 2, left oculomotor paresis and right spastic hypertonic hemiparesis, that improved in 6 months to GOS 3, with neurological rehabilitative therapy.
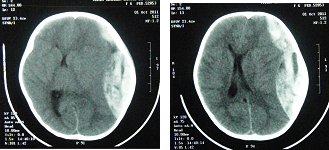
Figure 1 Brain Computed Tomography scan demonstrating extensive left epidural frontotemporoparietal hematoma, with midline shift of 15mm and subfalcine herniation.
Osteogenesis imperfecta (OI) is a genetically determined disease, in which structure and function of type 1 collagen are affected. Autosomal dominant is the most common inherited pattern, which may be less frequent, recessive. The change is determined by mutations in one of two genes coding for alpha chains of type 1 collagen in chromosomes 7 and 17, specifically in Loci COL1A1 and COL1A2, where the replacement of glycine by another amino acid is more frequent; mutation located in these Loci primarily determines the OI, but can also cause, among other diseases, Ehlers-Danlos syndrome types VIIA or VIIB.4,5 A large number of mutations have been described, but the correlation between specific genetic defect and produced phenotype is still not established.5
A classification by Sillence & Rimoin6 established in 1979, still used currently, distinguishes four major clinical phenotypes, based on the degree of bone deformities, with a variable prognosis. Recently, three distinct groups of moderate phenotypes were described: types V, VI, and VII.7-9 Type 3 OI is the most severe type among children who survive the neonatal period with wide variety degree of bone malformations and fractures. Characterized by structurally defective type I collagen these patients have many fractures starting early in life, many becoming wheelchair bound and shortened life expectancy.
The occurrence of coagulation disorders in 10-30% of patients increases the severity of trauma events, particularly intracranial, such as occurred with this child. The association of increased vascular fragility, decreased platelet retention, decreased levels of VIII factor, and deficient platelet aggregation induced by collagen are factors considered responsible for hemorrhagic complications in patients with OI.10-12 However, there is still no clear association about which subgroup of patients with OI will develop coagulopathy, even the genetic loci responsible for such.
There are no studies showing increased incidence of ischemic complication in OI more than the general population secondary to EDH. Neurosurgical implications in patients with OI are documented in the literature, mostly in isolated case reports. The neurosurgical conditions affecting these patients are related with bone dismorphisms like macrocephaly, basilar invagination and spinal deformities, development of hydrocephalus (communicating and non-communicating) and mainly the complications related with coagulation disorders like development of acute intracranial hematomas after minimal trauma and chronic subdural hematomas.13
Ischemia in middle and posterior cerebral arteries territories of this patient, diagnosed after cranial epidural hematoma drainage, could have occurred due mechanical compression of the vessels against cranioencephalic structures (one of primary mechanisms of ischemia related to the presence of extradural hematoma): outlying ischemia involving the territory irrigated by the posterior cerebral artery may have occurred due compression against the rigid tentorium edge due the massive hematoma and brain herniation to other side;12 and the temporoparietal ischemia by the direct mass effect of epidural hematoma on the underlying cerebral cortex, causing cerebral hypoperfusion and consequent peripheral cerebral infarction.14,15 Ulrich goes further stating that the abrupt reduction of hemoglobin in these patients would cause brain hypoflow and cellular hypoxia in those areas already suffering, highlighting the multifactorial aspect of this type of post traumatic complication, even in this patient. Besides, the author speculates that the elasticity of the arterial walls in childhood is certainly higher than in older patients, and these thin and flexible walls due to a lack of collagen fibers, give them a lower stability of the diameter of the vessel and its light in case of biomechanical stretch.16
A combination of vascular fragility and propensity to fractures can have significant consequences after a trauma considered trivial in patients with OI, particularly in type 3 OI children, but different than that one might conclude, although hemorrhagic cerebral complications are most frequent, post traumatic cerebral ischemia can also be found, increasing the scope and severity of secondary injuries related to head injuries in this group of patients.
None.
None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.