Journal of
eISSN: 2373-6410


Background: To determine the diagnostic effectiveness of (123I) ioflupane injection (DaTscan™) in patients with early, clinically uncertain parkinsonian syndrome (CUPS), 122 clinical trial subjects with CUPS who had experienced motor and non-motor signs and symptoms up to 5years prior to enrolment underwent DaTscan imaging.
Design/methods: Of the 122 subjects recruited from 19 centers (US and EU), 92 evaluable subjects were selected for analysis requiring baseline clinical diagnosis, DaTscan imaging results, and the reference 1 year post-scan clinical diagnosis. One-year post-scan clinical diagnosis and DaTscan imaging results were reference standards used for calculating the sensitivity, specificity, positive and negative predictive value (PPV and NPV) and diagnostic accuracy of the DaTscan imaging vs. clinical diagnosis. DaTscan imaging results were interpreted using blinded image evaluation (no access to clinical information) without consideration of the subject’s symptoms or clinical signs and categorized as Normal or Abnormal (grade 1, 2, or 3).
Results: At baseline, 75% of the subjects were in the early stages of CUPS (Hoehn and Yahr stages 0 to 2). Using 1-year post-scan clinical diagnosis as reference standard, specificity, PPV and NPV were better for the DaTscan imaging vs baseline clinical diagnosis. Using DaTscan imaging results as the reference standard, specificity, PPV and NPV of the 4-week clinical diagnosis (imaging results known by clinician) were better than baseline clinical diagnosis, sustained for 12-week and 1-year clinical diagnoses.
Conclusions: This study demonstrated high sensitivity and specificity, PPV, NPV and diagnostic accuracy of DaTscan imaging in diagnosis of early CUPS, comparing favorably to clinical diagnosis relative to final 1-year clinical diagnosis. One-year clinical diagnosis and DaTscan imaging results without clinical information were aligned either showing presence or absence of neurodegenerative disease. Study results suggest that DaTscan imaging is a useful adjunct in the diagnosis of early CUPS.
Keywords: DaTscanTM, Ioflupane I123 injection, Clinically uncertain parkinsonian syndrome, Sensitivity, Specificity, Diagnostic accuracy
AD, alzheimer’s disease; CI, confidence interval; CUPS, clinically uncertain parkinsonian syndrome; DAT, dopamine transporter; DLB, dementia with lewy bodies; ET, essential tremor; FN, false negative; FP, false positive; H&Y, hoehn and yahr; MSA, multiple system atrophy; non-PS, non-parkinsonian syndrome; NPV, negative predictive value; PD, parkinson’s disease; PPV, positive predictive value; PS, parkinsonian syndrome; PSP, progressive supranuclear palsy; SDD, striatal dopaminergic deficiency; SPECT, single-photon emission computed tomography; TN, true negative; TP, true positive
Parkinson’s disease (PD) is the most common parkinsonian syndrome (PS) and the second most prevalent neurodegenerative disorder after Alzheimer’s disease.1,2 The clinical diagnosis of PD can be uncertain, particularly when the disease is in its early stages, when signs and symptoms are mild or atypical or in tremulous PD cases.3 In cases of clinically uncertain parkinsonian syndrome (CUPS), single-photon emission computed tomography (SPECT) imaging with DaTscan™ (Ioflupane I123 Injection) is useful for improving diagnostic accuracy.4,5 DaTscan is a radiopharmaceutical that is approved in Europe (under the name DaTSCAN™) and the United States (under the name DaTscan™) for SPECT imaging to visualize the dopamine transporter in the brain.4-6 The clinical diagnosis of PD is particularly prone to error in the primary care setting. In a population-based study, Schrage et al found that at least 15% of patients who had received a diagnosis of PD in the community did not fulfill strict diagnostic criteria for PD and that 20% of the diagnosable cases of PD that had already come to medical attention had not been properly diagnosed.7 Meara et al.8 showed that 47% of 502 individuals who had received a presumptive diagnosis of PD from a general practice in the UK failed to fulfill the UK Parkinson’s Disease Society Brain Bank’s clinical diagnostic criteria for PD. Essential tremor was diagnosed in 50 of the 103 subjects (48%) in which Parkinsonism could not be demonstrated.
Marshall et al.9 found that PD tends to be over-diagnosed in clinically uncertain cases, when compared with the diagnosis that the same patient received 3years later. Studies have also shown that in roughly half of patients with CUPS, an initial presumptive diagnosis of PD was rejected after DaTscan imaging10–12 DaTscan imaging may be particularly useful for differentiating PD from essential tremor.13 General neurologists who do not specialize in movement disorders misdiagnose PD more often than movement disorder specialists. General neurologists changed the diagnosis in 75% and movement disorder specialists in 47% of CUPS cases after DaTscan imaging results became available and these changes remained over a 1-year observation period, suggestive of a high level of confidence associated with the revised diagnosis.12 DaTscan imaging visualizes striatal dopamine transporters; it correlates with dopaminergic degeneration in vivo and with subsequent autopsy examination (albeit for Lewy body disease in the Walker study).14
In 2012, Kupsch et al.12 published a phase 4 study in which subjects with CUPS at baseline were randomly assigned to either DaTscan imaging or no imaging between baseline and week 4.12 This study showed that the availability of DaTscan imaging results often led to a significant change in diagnosis starting at 4 weeks in 45% of patients vs 9% of the controls who did not receive DaTscan, an increase in the physician’s confidence of the revised diagnosis, and a significant change in the clinical management at 12 weeks in 50% of patients vs 31% of the controls.
In the current report, we estimate the sensitivity, specificity, positive and negative predictive values, and diagnostic accuracy of DaTscan imaging, which were not analyzed and reported in the Kupsch et al.12 publication. Ideally, the gold standard for establishing those test characteristics would be a neuropathology examination at autopsy, but that approach is unfeasible, as few cases of CUPS come to autopsy. In support of DaTscan imaging findings, according to the Colloby et al.15 2012 publication in which dopaminergic neurons were counted post-mortem, there was a statistically significant correlation between DaTscan imaging and dopaminergic neuronal density in substantia nigra for putamen and the striatum.15 In this study, we used two reference standards the clinical diagnosis at 1year post-scan (with DaTscan imaging results available) and the exploratory reference standard, the DaTscan imaging results (blinded to clinical information) (Figure 1).
All study protocols, amendments, and informed consent documents were approved by the appropriate ethics committees or institutional review boards prior to study start. Prior to the initiation of any study procedures, informed consent was obtained from participating patients. This study was conducted in full accordance with the Declaration of Helsinki (World Medical Association), the International Conference on Harmonization consolidated guideline E6 Good Clinical Practice and applicable national and local laws and regulations.
Data source
This study is a new efficacy analysis of data from a global (USA and EU), phase 4, open-label clinical utility, multicentre(19) study of SPECT imaging with DaTscan in cases of CUPS.12 The study subjects had CUPS, which was defined as parkinsonian signs that had arisen within the past 5years but were mild, atypical and/or non-progressive and/or had responded poorly to levodopa. In particular, a subject might have only one of the cardinal signs of Parkinsonism (with or without asymmetry) or might have two signs without fatigable bradykinesia. Individuals with a certain or established movement disorder or a known cause for tremor (e.g. hyperthyroidism) were excluded, as were individuals for whom a specific diagnosis of PD or another PS (progressive supranuclear palsy (PSP) or multiple system atrophy (MSA) was confirmed. Medications such as amphetamine, benztropine, bupropion, cocaine, mazindol, methylphenidate, phentermine and sertraline that were known to compete with the DaTscan radiopharmaceutical for binding to dopamine transporters had to be discontinued for at least 5 half-lives prior to DaTscan administration.
The enrolled subjects (n=273) underwent a complete clinical evaluation to establish a baseline clinical diagnosis and were randomly assigned to undergo either SPECT imaging with DaTscan (n=135) or no imaging (n=138) within 4 weeks of baseline. Clinical evaluators were 7 general neurologists and 12 movement disorder specialists. DaTscan images were visually interpreted via a decentralized approach by using one local nuclear medicine reader per centre, each of whom was an expert in neuroimaging and blinded to the subjects’ clinical information. Subjects were seen for additional clinical visits at weeks 4 (Visit 2) and 12 (Visit 3) and at 1year (Visit 4). For subjects who had undergone DaTscan imaging (undertaken at Nuclear Medicine visit between Visit 1 (baseline) and Visit 2), the imaging results were available to the clinician at Visits 2-4. Thus, the DaTscan imaging results could influence the clinician’s diagnoses at weeks 4 and 12 and at 1year after baseline.
Interpretation of DaTscan imaging results
DaTscan SPECT were collected and reconstructed as transaxial, parallel-slice images per standardized imaging protocol (Kupsch et al. 2012 for description of the imaging methodology).12 The interpretation of the DaTscan imaging results was based purely on a blinded image evaluation (no access to clinical information), without consideration of the subject’s symptoms or clinical signs. DaTscan imaging results were categorized as Normal or Abnormal, and if Abnormal, grade 1, 2, or 3 (Figure 1).15
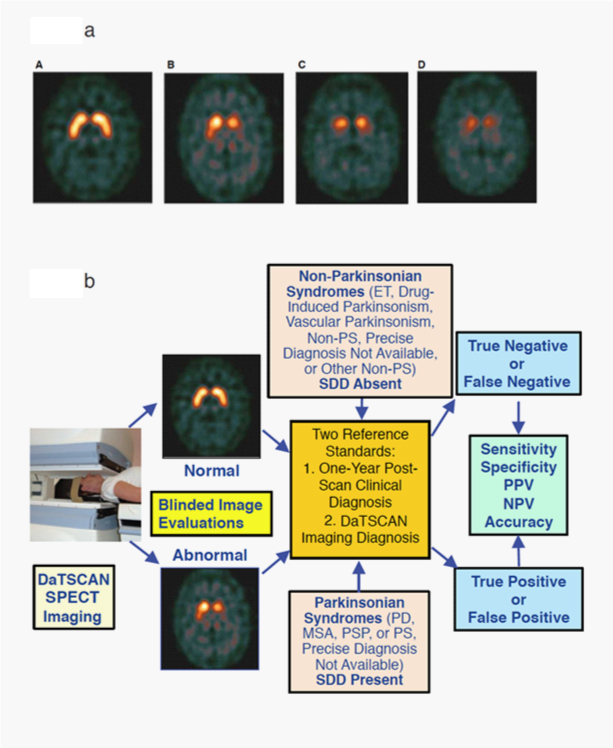
Figure 1 Examples of DaTscan Images and DaTscan Efficacy Analysis Path.
1A. Examples of DaTscan transaxial images and grading subtypes, Normal, Abnormal Grade 1, Abnormal Grade 2, Abnormal Grade 3.29
Normal: Characterized by two symmetric comma- or crescent-shaped focal regions of activity mirrored about the median plane. Striatal activity is distinct, relative to surrounding brain tissue.
Abnormal grade 1: Activity is asymmetric, e.g. activity in the region of the putamen of one hemisphere is absent or greatly reduced with respect to the other. Activity is still visible in the caudate nuclei of both hemispheres resulting in a comma or crescent shape in one and a circular or oval focus in the other. There may be reduced activity between at least one striatum and surrounding tissues.
Abnormal grade 2: Activity is absent in the putamen of both hemispheres and confined to the caudate nuclei. Activity is relatively symmetric and forms two roughly circular or oval foci. Activity of one or both is generally reduced.
Abnormal grade 3: Activity is absent in the putamen of both hemispheres and greatly reduced in one or both caudate nuclei. Activity of the striata with respect to the background is reduced.
1B. DaTscan efficacy analysis path with representative normal and abnormal images.
ET: essential tremor; MSA: Multiple System Atrophy; Non-PS: Non-Parkinsonian Syndrome(s); NPV: Negative Predictive Value; PD: Parkinson’s Disease; PPV: Positive Predictive Value; PS: Parkinsonian Syndrome(s); PSP: Progressive Supranuclear Palsy; SDD: Striatal Dopaminergic Deficiency.
Study populations
This new efficacy analysis includes data from the subset of subjects who underwent SPECT imaging with DaTscan in the phase 4 trials.12 At baseline, all of these subjects had a clinical diagnosis of CUPS. For each analysis, the subject’s clinical diagnoses were used to classify each subject into one or both of two populations: a narrow population and a broad population to differentiate diagnostic performance/efficacy of DaTscan imaging between the two populations. The narrow population in each analysis included only those subjects who were diagnosed as having a specific diagnosis, either a specific PS (PD, MSA, or PSP) or a specific non-parkinsonian syndrome (non-PS) (only essential tremor (ET)). The results of the DaTscan imaging would be expected to be positive/abnormal (i.e. to have a striatal dopaminergic deficiency (SDD)) for the subjects with a PS or negative/normal (no SDD) for subjects with a non-PS. The broad population included subjects both with the narrow (specific) list of diagnoses summarized above, but also subjects with non-specific diagnoses, such as "PS, precise diagnosis not defined" and "non-PS, precise diagnosis not defined." Table 1 summarizes all 1-year diagnoses for subjects in this study and indicates which diagnoses are contained in the narrow and broad categories, focusing on DaTscan results from the PS subjects.
Statistical methods
A diagnosis of a PS was considered to be an abnormal DaTscan imaging result with SDD present. A diagnosis of a non-PS (ET) was considered to be a normal DaTscan imaging result with SDD absent. A diagnosis arrived at by any means was considered to be true if it agreed with the reference standard and false if it did not. Thus, each diagnosis was classified as true positive (TP), false positive (FP), true negative (TN) or false negative (FN) relative to the reference standard being used (see Results and Discussion). The 1-year post-scan clinical diagnosis was used as the reference standard for judging the DaTscan imaging results and the clinical diagnoses at baseline, 4weeks, and 12 weeks. However, subjects in this study had experienced motor and non-motor signs and symptoms and were under physician’s observation for up to 5years prior to DaTscan imaging, which provided sufficient information for the 1 year clinical diagnosis post-scan to be used as a reference standard. In a separate analysis, the DaTscan imaging results (i.e. positive or negative for SDD) were used as the exploratory reference standard for judging the clinical diagnoses at all time points.
Test characteristics, relative to each reference standard, were calculated in accordance with standard definitions: Sensitivity=TP/ (TP+FN); Specificity=TN/ (FP+TN); Accuracy=(TP+TN)/ (TP+TN+FP+FN); PPV=TP/ (TP+FP); NPV=TN/ (FN+TN). Subjects were omitted from a particular calculation if their results were missing (e.g. if their week 4 or week 12 clinical diagnosis was missing).
We used McNamara’s test with Yates’ correction for continuity to compare the sensitivity and specificity of a pair of predictors for the same reference standard (for example, comparing the sensitivity and specificity of DaTscan imaging to the sensitivity and specificity of baseline clinical diagnosis, using 1-year post-scan clinical diagnosis as the reference standard).16,17 The Leisenring et al.17 method was used to compare the PPV and the NPV of a pair of predictors for the same reference standard 17. No P-values were calculated for diagnostic accuracy because there are no appropriate statistical methods for comparing accuracy with correlated data 18.
Analysis populations
In the phase 4 trial, from 135 subjects enrolled in imaging group, 122 subjects underwent DaTscan imaging.12 Of these subjects, 12 had a missing clinical diagnosis at 1 year, 3 had inconclusive Visit 4 diagnoses, 10 had missing DaTscan (normal/abnormal) imaging results because of protocol violations, 4 had missing Visit 4 clinical diagnoses and missing DaTscan imaging results and 1 had a missing Visit 4 clinical diagnosis and a non-evaluable DaTscan imaging result, yielding 92 evaluable subjects for analysis requiring baseline clinical diagnosis, DaTscan imaging results and the reference 1-year post-scan clinical diagnosis. Because of the (often) expected inconclusive diagnoses after 1 year (keeping in mind that these are early CUPS patients), only conclusive cases with specific diagnoses were selected for our analyses.
In the current report, the numbers of subjects included in specific analyses depended both upon whether or not subjects were included in the broad or narrow populations and on the nature of the analysis. A total of 92 subjects were included in the broad population for comparison of baseline clinical diagnosis or DaTscan imaging with the reference 1-year post-scan clinical diagnosis and 98 evaluable subjects were included to compare the 4-week and 12-week clinical diagnoses with the 1-year clinical diagnosis however, the number of subjects varied for the different analyses. When the DaTscan imaging result was used as the exploratory reference standard to examine the clinical diagnoses at each visit, the number of subjects varied between 92 and 100, depending on the number of evaluable diagnoses at each visit. According to the definitions of the study populations, the larger broad population included all subjects in the narrow population (67 subjects with diagnoses of PD, MSA, PSP, and ET). However, at baseline, 8 of the 67 subjects did not fit the criteria of the narrow population and were excluded, resulting in 59 evaluable subjects for this narrow group evaluation who had both DaTscan imaging and narrow clinical diagnosis at Visit 4. Therefore, the number of subjects in the two analyses of the narrow population using 1 year post-scan clinical diagnosis as reference standard were 59 and 40 (had narrow category clinical diagnoses at both Visit 1 and Visit 4).
Table 1 shows the frequency of clinical diagnoses at 1-year post-scan and whether or not the subjects with a particular diagnosis were included or excluded in the relevant population group. Of the 67 subjects in the narrow population, 42 subjects had a diagnosis of PD, 5 subjects had a diagnosis of another PS (MSA or PSP), and 20 subjects had a diagnosis of a non-PS (ET). The broad population also included 7 subjects who had a PS that could not be precisely classified, as well as 28 subjects with a non-PS other than ET, of whom 2 had drug-induced parkinsonism; 3, vascular parkinsonism; 15, other non-PS and 8, "precise non-PS not available."
Table 2 shows the baseline demographic characteristics for the 92 subjects in the broad population who had baseline clinical diagnosis, DaTscan imaging results and 1-year post-scan clinical diagnoses. Of these subjects, 50 (54.3%) were male. The mean age was 67.9 years, and the mean time since onset of symptoms was 2.61 years. The mean score for the Mini-Mental State Examination was 28.6. Tremor was the most common first parkinsonian sign or symptom, occurring in 69 (75.0%) of subjects. At baseline, 74 (80.4%) were taking at least one concomitant medication for central nervous system disease, with 50 (54.3%) taking anti-PD drugs.
Table 3 describes the results of the baseline neurologic examination for subjects in the broad population who had both DaTscan imaging results and 1-year post-scan clinical diagnoses. Tremor was a common finding: 62 (67.4%) definitely experienced tremor and 9 (9.8%) possibly experienced tremor. Bradykinesia was also common 26 (28.3%) had definite bradykinesia and 36 (39.1%) had possible bradykinesia. Rigidity was also common 32 subjects (34.8%) definitely had rigidity and 28 (30.4%) had possible rigidity. At baseline, 12 subjects (13%) reported neuropsychiatric symptoms and 12 subjects (13%) reported sleep disorders. The principal reasons for the diagnosis of CUPS at baseline were atypical signs (52 subjects, 56.5%) or the presence of only 1 of the 3 cardinal signs of Parkinsonism (35 subjects, 38.0%). At baseline, most of the subjects were in the early stages of a PS (Hoehn and Yahr (H&Y) stages 0 to 2). Thirty-nine (42.4%) were in stage 1.0 (unilateral disease) and 30 (32.6%) were in stage 2.0. In the broad population of 92 subjects, 40 subjects had normal DaTscan images. Of the 52 subjects with an abnormal DaTscan image, Abnormal types 1 and 2 were most commonly associated with H&Y stage 1 (42.3%) and H&Y with stage 2 (51.9%) (Table 4).
Reference standard:
Clinical diagnosis at 1 year post-scan in the broad population: Table 5 (Parts I and II) shows the sensitivity, specificity, accuracy, PPV and NPV for the baseline clinical diagnosis and the baseline DaTscan imaging results, as compared with the clinical diagnosis at 1 year post-scan, for subjects in the broad population. The other criteria for inclusion in the specific analyses are stated in the table footnotes. When the clinical diagnosis at 1 year post-scan was used as the reference standard, the DaTscan imaging had a sensitivity of 0.9388, a specificity of 0.9535, a diagnostic accuracy of 0.9457, and a PPV of 0.9583 and NPV of 0.9318. Although the DaTscan imaging and the baseline clinical diagnosis had similar sensitivity and NPV, the DaTscan imaging had better specificity (P=0.0005) and PPV (P<0.0001) than did the baseline clinical diagnosis.
The baseline clinical diagnosis was made before the DaTscan imaging was performed. However, the results of the DaTscan imaging were available to the clinicians who made the clinical diagnosis at weeks 4 and 12 (Table 5 (Parts III and IV)), which shows the sensitivity, specificity, accuracy, PPV, and NPV for the clinical diagnosis that was made at 4 weeks and 12 weeks when compared with the clinical diagnosis reached at 1 year post-scan. The sensitivity, specificity, PPV and NPV were higher at week 4 than at baseline, all of which reached statistical significance. Sensitivity increased from 0.9200 to 0.9815, specificity increased from 0.5238 to 0.9318 (P=0.0007), diagnostic accuracy increased from 0.7391 to 0.9592, PPV increased from 0.6970 to 0.9464 (P=0.00003) and NPV increased from 0.8462 to 0.9762 (P=0.0335). High sensitivity, specificity, accuracy, PPV, and NPV at 12 weeks were comparable to those at 4 weeks.
Reference standard:
Clinical diagnosis at 1 year post-scan in the narrow population: Although all of the subjects had CUPS at baseline, 40 of the subjects received a clinical diagnosis of either PS (PD, MSA, or PSP) or no PS (ET) (Table 6 (Part I) at Visit 4 (1 year)). When clinical diagnosis at 1 year post-scan was used as the reference standard, their baseline clinical diagnosis had a sensitivity of 0.9583, a specificity of 0.8125, a diagnostic accuracy of 0.9000, a PPV of 0.8846, and a NPV of 0.9286 (Table 6 (Part I)). All test characteristics for the baseline clinical diagnosis were better for subjects in the narrow population than for subjects in the broad population (Table 4 (Part I) vs. Table 6 (Part I)).
At 1 year, 59 of the subjects had a DaTscan imaging results and specific 1-year clinical diagnoses, for which they were included in the narrow population. When the clinical diagnosis at 1 year post-scan was used as the reference standard, the DaTscan imaging for this population had a sensitivity of 0.9524, a specificity of 1.0000, a diagnostic accuracy of 0.9661, a PPV of 1.0000 and a NPV of 0.8947 (Table 6 (Part II)). However, these test characteristics were not significantly different from those for baseline clinical diagnosis (Table 6 (Part I)) in this population.
Reference standard
DaTscan imaging results in the broad population: Table 7 shows the values for sensitivity, specificity, PPV, NPV, and diagnostic accuracy for the clinical diagnosis at baseline, week 4 (Visit 2), week 12 (Visit 3), and 1 year (Visit 4), using DaTscan imaging results as the reference standard in the broad population. Clinical diagnosis at baseline was made before the DaTscan imaging was performed. Clinical diagnoses made at week 4 and later were made with knowledge of the DaTscan results and compared to the DaTscan imaging results alone (i.e. Abnormal/PS or Normal/non-PS) as a reference standard. Changes from baseline to subsequent visits were statistically significant for specificity: 0.4773 at baseline to 0.9184 at 4 weeks (P=0.0001); remained high at 12 weeks (0.9375: P=0.0001) and at 1 year (0.9318: P= 0.0001).
The other test characteristics also improved from baseline to 4 weeks: sensitivity increased from 0.8400 at baseline to 0.9804 at 4 weeks (P=0.0736), PPV from 0.6462 at baseline to 0.9259 at 4 weeks (P=0.000001), NPV increased from baseline 0.7241 to 0.9783 at 4 weeks (P=0.009) and accuracy from baseline 0.6702 to 0.9500 at 4 weeks. These test characteristics were consistent with values at 12 weeks and at 1 year. In summary, when the DaTscan imaging results became available at week 4, the clinical diagnosis was often changed to more closely match the DaTscan imaging results. The clinical diagnosis at 12 weeks and 1 year tended to remain in agreement with the baseline DaTscan imaging results. Figure 2 displays the diagnostic performance results obtained against both reference standards for subjects in the broad population.
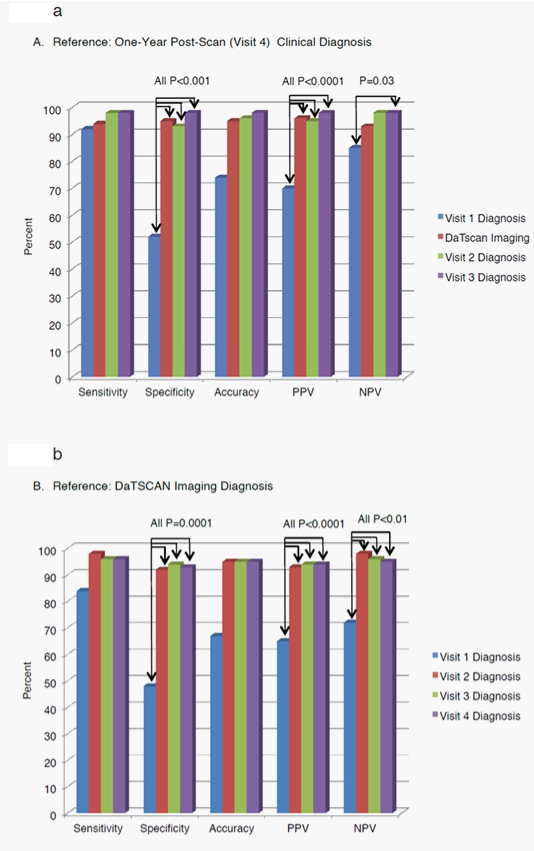
Figure 2 Efficacy Test Results Across All Study Visits Using Two Reference Standards; One-Year Post-Scan Clinical Diagnosis (A) and DaTscan Imaging Diagnosis (B) in the Broad Populations
DaTscan imaging test characteristics
DaTscan imaging had high sensitivity, specificity, PPV, NPV and diagnostic accuracy for predicting the clinical diagnosis at 1 year after the baseline visit for subjects with early CUPS, which represents about one-fifth of patients with PS 7. These test characteristics were high for subjects who received a specific diagnosis of PS (PD, MSA, or PSP) or non-PS (ET) at the end of the year (the narrow population) as well as for subjects diagnosed from a broader list of diagnoses (the broad population) including drug-induced parkinsonism, a non-specified PS, or non-PS. These findings reflect a role of dopamine transporter imaging as a diagnostic adjunct as part of the patient’s work-up to diagnose uncertain movement disorders.
Baseline clinical diagnosis and the DaTscan imaging results had similar sensitivity and NPV using the 1-year post-scan clinical diagnosis as reference standard. However, baseline clinical diagnosis had a lower specificity and lower PPV, which is consistent with earlier findings that clinicians often misdiagnose or over-diagnose PS in clinically uncertain cases and change their diagnosis when given access to the DaTscan imaging results, which can integrate pathophysiological information about presence or absence of SDD in doubtful cases 12,19. This study showed that clinicians often change their clinical diagnosis to bring it into line with the DaTscan imaging results, and that the clinical diagnosis tends to remain in agreement with the baseline DaTscan imaging results over time. The tendency of the clinical diagnosis to converge with the results of the DaTscan imaging was found not only for subjects who went on to have a specific diagnosis of PS or non-PS (the narrow population) at 1 year, but also in subjects with a broad list of 1-year diagnoses (the broad population), including diagnoses, such as "PS, precise diagnosis not available" or "non-PS, precise diagnosis not available."
After clinicians had acquired the DaTscan imaging results, only a few of the clinical diagnoses of a PS at weeks 4 and 12 were classified as false-negatives or false-positives. Revised clinical diagnoses were generally maintained during the 1-year follow-up after the clinical diagnosis had been changed following availability of DaTscan imaging results.
The clinical diagnosis at week 4, which was based on the medical history and physical and neurologic examination with DaTscan imaging results available, had a high specificity and PPV when the clinical diagnosis at 1 year post-scan was used as the reference standard and a high specificity, PPV and NPV when the DaTscan imaging results were used as the exploratory reference standard (i.e. without access to medical history or the physical and neurological examinations). Thus, the use of DaTscan imaging may improve the presumptive diagnosis of parkinsonism in subjects with CUPS, which is an important in consideration of the previously observed clinical utility and impact of this diagnostic test on patient treatment and clinical management.3,12,19,20
The outcomes from this report are consistent with those of the Marshall study in which the clinicians had a low specificity for baseline clinical diagnosis without access to DaTscan imaging results (blinded study design). Specificity substantially improved and approached consistency with the DaTscan imaging results only at the time of the 3 year clinical diagnosis.9 Thus, use of DaTscan imaging results may enable an early, accurate diagnosis. The current study, as it was not a regulatory trial and not blinded for its duration, complements the Marshall Study results and buttresses the understanding of the impact of diagnostic testing on the clinical decision-making process when DaTscan imaging is combined with clinical information. Once the clinicians have seen the scan results and revise their initial diagnosis, they tend not to return to their original diagnosis. Thus, despite the differences in the trial designs (blinded in Marshall’s registration study9 vs open-labeled in Kupsch’s clinical utility study),12 the results of this study are somewhat consistent with the Marshall study, in that both studies demonstrated a high sensitivity and a low specificity of initial clinical diagnosis, suggestive of a trend for over diagnosis of PS/PD in clinical practice, especially in subjects with early and uncertain PS. DaTscan imaging is now a regulatory-approved diagnostic test incorporated in the clinical practice, and thus it was unnecessary, in fact unethical, to withhold information in decision-making for ongoing patient management. While utilization of an open-label design might be viewed as a limitation of Kupsch’s study, it was designed to resemble clinical practice to further understand the impact of DaTscan imaging on clinical management and thereby to assess the impact on the clinical diagnoses over time based on access to DaTscan imaging results.
Although the sensitivity and specificity of DaTscan imaging for the diagnosis of PS are high, and closely approach 100%, they are only a "snap shot in time".19 Also, the inter-rater reliability for DaTscan images in clinical trials has been less than 100%.13 Perl mutter and Eidelberg found that some patients with negative DaTscan imaging results were diagnosed with PD or another PS within a few years 21. Nevertheless, the overall error rate appears to be low. A UK National Centre of Excellence for PD audited results of DaTscan imaging vs clinical diagnosis in 731 patients over a 9-year period.19 During that period, there appeared to be no issues in establishing the final clinical diagnosis to be used as a reliable reference standard. The audit yielded only 5 false-positive and 2 false-negative DaTscan results. In that setting, DaTscan imaging, when compared to the final clinical results had specificity of 98.6%, sensitivity of 99.4%, positive predictive value of 98.7%, negative predictive value of 99.4%, and overall accuracy of 99.1%, which is similar to our findings.19 Furthermore, an autopsy study of patients with Lewy Body dementia (and similarly in patients with PD, per personal communication with the principal investigator Dr. Z. Walker) reported that DaTscan imaging correlated well with autopsy findings supporting the use of DaTscan SPECT for detection of dopaminergic degeneration in vivo.14
Clinical implications for use of dopamine transporter (DaT) imaging
The results of this study showing high specificity and the predictive value of DaTscan imaging support the use of this diagnostic test as part of a patient enrichment strategy in clinical trials of investigative medications for PD and other neurodegenerative disorders. This potential application is in accord with the recent FDA draft guideline on enrichment strategies for clinical trials to support approval of human drugs and biological products.22 Although the guide does not specifically mention DaTscan imaging, the Parkinson Progression Marker Initiative (PPMI) is currently implementing DaTscan enrichment as a part of study inclusion criteria and during a 5-year follow-up seeking to establish PD biomarker progression.23 In addition, the FDA supported Coalition against Major Diseases (CAMD) seeks to qualify a DAT imaging biomarker for patient enrichment in PD clinical trials.24 We also note that this diagnostic test has achieved a wide acceptance within the PD community, major professional societies, and organizations. Based on current guidelines and consensus statements, DaTscan imaging is supported as a useful and validated diagnostic tool in the 2013 EFNS/MDS-ES guideline (Category A). The Society of Nuclear Medicine, the UK’s National Institute for Health and Clinical Excellence (NICE) 2006 guidance and the Scottish Intercollegiate Guidelines Network (SIGN) which are supported by our trial results.25–28
The analyses carried out in this report in an early CUPS population demonstrated the high diagnostic test characteristics of DaTscan imaging, as shown by consistent improvement in clinical diagnoses after imaging results became available and were integrated with the clinical information. Performance of DaTscan imaging compares favorably to final clinical diagnosis at 1year. When the clinical diagnosis at 1year post-scan was used as the reference standard, the specificity, PPV and NPV were better for the DaTscan imaging than for the baseline clinical diagnosis. When the DaTscan imaging was used as the exploratory reference standard, the specificity, PPV and NPV for the 4-week clinical diagnosis (when DaTscan imaging results were available) were also better than baseline clinical diagnosis and were sustained at 12-week and 1-year. Results of analyses using both reference standards were aligned either showing presence or absence of neurodegenerative disease.
Results that were observed in the phase 4 study (changes in diagnosis, confidence of diagnosis, and clinical management)12 were complemented by new diagnostic effectiveness results (sensitivity, specificity, accuracy, PPV, and NPV) in the current report and suggest that the observed changes in diagnosis were in the right direction (i.e. better agreement between final clinical diagnosis and DaTscan imaging results). Study results suggest that DaTscan imaging is a useful adjunct in the diagnosis of early CUPS.
Strengths
Limitations
The authors wish to thank the investigators for the original trial that provided the data for this analysis: Andreas Kupsch, Ana Garcia, Doreen Gruber, Henriette Krug, Elmar Lobsien, Thomas Totenberg at Charite Campus Virchow, Department of Neurology, and Michail Plotkin at Department of Nuclear Medicine, Berlin, Germany; Carsten Buhman, Ute Hidding at Ambulanzzentrum des UKE GmbH Bereich Neurologie University Hospital Hamburg-Eppendorf, Hamburg, Germany; Helen Roberts, Vanessa Pressly at Southampton General Hospital, Department of Geriatric Medicine, Southampton, UK; Nin Bajaj, Vamsi Gontu, at Derbyshire Royal Infirmary Neurology Department, Derby, UK; Urs Pato at Inselspital Bern Neurologische Universitatsklinik and Poliklinik, Bern, Switzerland; Tove Hauge, Berd Mueller at Nordmore og Romsdal HF Molde Hospital, Neurology Department, Molde, Norway; Pierre Charpentier, Bachir Makki at Centre Hospitalier de Bethune, Neurology Department, Bethune, France; Antonio Tartaglione, Elena Carabelli, Lucia Baruzzo, Federica Vivarelli, Department of Neurology and Antonella Montepagani, Department of Nuclear Medicine, Ospedale S. Andrea, La Spezia, Italy; Juan Carlos Martinez Castrillo, Maria Eugenia Rioja, Jaime Masjuan at Hospital Ramón y Cajal de Madrid, Madrid, Spain; Jose Balseiro Gomez at Hospital Universitario de Getafe, Neurology Department, Madrid, Spain; Maria Dolores Escriche Jaime, Andreas Serena Puig at Hospital Meixoeiro de Vigo, Vigo, Spain; Jan Aasly, Harald Johnsen at St. Olavs Hospital HF, Neurology Department, Trondheim, Norway; Jesper Clausen at KAS Glostrup Hospital, Neurology Department, Glostrup, Denmark; Donald Grosset, Katherine Grosset at Glasgow Southern General Hospital, Glasgow, UK; Deborah Burke, Theresa McClain at University of South Florida, Tampa, Fl, USA; Mark Stacy, Burton Scott, Ralph Coleman, Julia Johnson at Duke University Movement Disorders Center, Durham, NC, USA; Danna Jennings, David Russell at Institute for Neurodegenerative Disorders, New Haven, CT, USA and Frederick Weiland, Nadine Yassa, Penny Vande Streek, Nicklesh Thakur at Sutter Health, Roseville, CA, USA. The authors would also like to thank Marjorie Winters of Winfield Consulting for her assistance in contributing to draft contents; Earl W. Henry, Statistician Contractor for GE Healthcare, for his review of the paper and valuable comments; and Fred E. Longenecker, Regulatory Affairs, GE Healthcare for his review of the paper and valuable comments. This analysis was funded by GE Healthcare.
RA Hauser: Allergen, Angiochem, Adamas Pharmaceuticals, Inc, AstraZeneca, Boehringer Ingelheim Pharm Inc, Chelsea Therapeutics, Civitas Therapeutics Inc, Clarion Healthcare, EMD Serono, GE Healthcare, Genzyme, GlaxoSmithKline, GSK, IMPAX, Ipsen, Lundbeck, Merck Serono, Merck Sharpe & Dohme, Novartis, Novartis/Orion Pharmaceuticals, Novan Pharmaceuticals, Inc, Santhera Pharmaceuticals Ltd., Schering-Plough, Straken Pharmaceuticals, Synosia, Solvay, TEVA Neuroscience, TEVA Pharmaceuticals, UCB, Xenoport, Inc.
ID Grachev: Employee of GE Healthcare, Princeton, NJ, USA.
K Marek: Ownership in Molecular Neuroimaging, LLC; Consultancy agreements: Pfizer, GE Healthcare, Merck, Lilly, BMS, Piramal, Prothena, Neurophage, nLife, Roche.
J Seibyl: Equity in Molecular Neuroimaging, LLC; Consultancy agreements: Bayer Healthcare, GE Healthcare, and Piramal Health.
A Kupsch: Honoraria: Allergen, Boehringer Ingelheim, Ipsen Pharma, Lundbeck, Medtronic, Merck, Merz Pharmaceuticals, Orion, UCB; Grants: German Research Council, German Ministry of Education and Research.
M Plotkin: None
T Lin: Employee of H2O Clinical LLC, Cockeysville, MD, USA.
N. Bajaj: Honoraria: UCB, Teva, Genus, GE Healthcare; Grants: MRC, Parkinson’s UK; Shares: None.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.