Journal of
eISSN: 2377-4282


Research Article Volume 2 Issue 5
Faculty of Chemistry, California South University, USA
Correspondence: Heidari A, Faculty of Chemistry, California South University, 14731 Comet St. Irvine, CA 92604, USA
Received: October 28, 2015 | Published: December 19, 2015
Citation: Heidari A, Brown C (2015) Study of Composition and Morphology of Cadmium Oxide (CdO) Nanoparticles for Eliminating Cancer Cells. J Nanomed Res 2(5): 00042. DOI: 10.15406/jnmr.2015.02.00042
The effects of substrate temperature on the structural, optical, medical and electrical characteristics of Cadmium Oxide (CdO) films are investigated in the current study. Films are deposited on glass substrate at different temperatures, ranged between 450–600 ºC, using Spray Pyrolysis Technique. According to X–Ray Diffraction (XRD), it is found that the films are polycrystalline and Cadmium Oxide (CdO) is of a hexagonal wurtzite structure which the preferred direction of its crystal plane is (002). The maximum optical transmittance of films is more than 85%. The size of crystallites is calculated with the maximum value in 500º C. Furthermore, Cadmium Oxide (CdO) nanoparticles were synthesized in deionized water by laser ablation of Cadmium plate. In the current paper, the effects of laser pulse energy and wavelength on the characteristics of Cadmium Oxide (CdO) nanoparticles were studied. Analyses of Transmission Electron Microscope (TEM) were confirmed that size distribution of Cadmium Oxide (CdO) nanoparticles is reduced by increase in laser pulse energy. Fluorescence spectrum of Cadmium Oxide (CdO) nanoparticles shows violet emission accompanied by blue and green band. UV emission with high intensity shows that nanostructure of Cadmium Oxide (CdO) is of a little deficiency. Also, the current study aims to study the linear and non–linear optical characteristics of Cadmium Oxide (CdO) nanoparticles and their applications. Firstly, fluorescence spectrum, as a linear characteristics of Cadmium Oxide (CdO), as well as second harmonic generation and two photons absorption, as non–linear characteristics of Cadmium Oxide (CdO), are studied. Then, some aspects of medicinal and pharmaceutical applications of Cadmium Oxide (CdO) nanoparticles for eliminating cancer cells such as the effect of Cadmium Oxide (CdO) nanoparticles on DNA of human cancer cells and the interaction of Cadmium Oxide (CdO) nanoparticles with DNA of cancer cells are originally investigated. It should be noted that the range of considered parameters are separately clarified and explained in each of related sections (Figure 1).
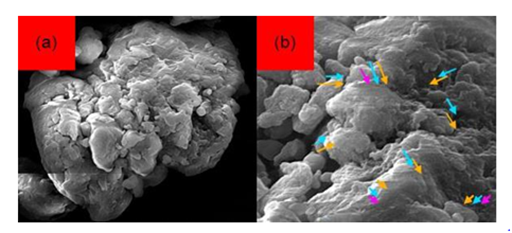
Figure 1 Scanning Electron Microscope (SEM) images of Cadmium Oxide (CdO) nanoparticles with 50000x zoom.
Keywords: Cadmium oxide (CdO) nanoparticles, Laser ablation, Optical parameters, X-Ray Diffraction (XRD), Transmission Electron Microscope (TEM), Laser pulse energy, UV emission, Pulsed Laser Deposition (PLD), Cancer cells, Composition, Morphology, Medical characteristics, Dynamic Light Scattering (DLS), Scanning Electron Microscope (SEM)
CdO, Cadmium Oxide; XRD, X–Ray Diffraction; TEM, Transmission Electron Microscope; PLD, Pulsed Laser Deposition; DLS, Dynamic Light Scattering; SEM, Scanning Electron Microscope
Semiconductor films of Cadmium Oxide (CdO) nanostructures have a direct and wide band gap (4.05 eV) and are of unique applicable characteristics for gas sensors, solar cells, laser, Spintronics and etc. Cadmium Oxide (CdO) is polycrystalline and is of wurtzite structure and is an n–type semiconductor .1-28
Cadmium Oxide (CdO) films have been produced using different techniques such as magnetron sputtering .29,30 Pulsed Laser Deposition (PLD) .31-39 Spray Pyrolysis Technique .40 and sol–gel .31-55 Spray Pyrolysis Technique is one of the least expensive and most simple methods for producing Cadmium Oxide (CdO) films. The technique is one of the most industrial methods for deposition of Cadmium Oxide (CdO) films and other transparent conductor oxides .56-87
Cadmium Oxide (CdO) is a unique chemical which is of both semiconductor and piezoelectric characteristics. Compared to other semiconductors, Cadmium Oxide (CdO) has higher Exciton binding energy (75 meV) and gap energy of about 4.05 (eV) .88-108 Various chemical methods have been reported for synthesizing the nanostructure of Cadmium Oxide (CdO). Most of the methods are so expensive and complex, especially for controlling the size of particles and uniformity of their size. However, laser ablation is one of the emerging methods for synthesizing the nanostructure of Cadmium Oxide (CdO) .109-121 The most important characteristic of this method is that it is possible to control the size of produced material by changing various parameters such as laser wavelength, laser pulse duration, pH of solution, added surfactant and temperature .121-153
In the current test, shaping up of Cadmium Oxide (CdO) nanoparticles by laser ablation is studied and the effects of laser pulse energy and wavelength on size of nanoparticles and optical and medical characteristics of Cadmium Oxide (CdO) in room temperature are investigated. Cadmium Oxide (CdO) is a semiconductor of group II–VI with direct band gap of about 4.1 (eV) in room temperature and exciton binding energy of about 75 (meV) .154-183 Exciton recombination of Cadmium Oxide (CdO) nanoparticles leads to UV emission of about 415 nanometers .184-190 Many researchers are interested in this characteristic of Cadmium Oxide (CdO) to be used in LEDs with short wavelength .191,192 Due to sharp exciton transition of Cadmium Oxide (CdO), it can be used in semiconductor lasers. Moreover, it can be used as transparent electrodes of solar cells .193-198
Comparing with nanoparticles of other toxic semiconductors, Cadmium Oxide (CdO) nanoparticles are of lowest toxicity .199, 200 Today, a great part of multivitamin pills and dietary supplements contain Cadmium .201-204 In addition, Cadmium Oxide (CdO) presents in various cosmetics and anti–solar creams. Hence, Cadmium Oxide (CdO) can be considered as a chemical compatible with the body .205-207 Further, this chemical is an appropriate option for bioapplications due to its good optical characteristics such as fluorescence, high resolution second harmonic generation and two photons emissions.
Cadmium Oxide (CdO) nanoparticles are of anti–cancer properties. Because of their unusual optical, chemical, photo electrochemical and electrical properties, Cadmium Oxide (CdO) nanoparticles are interesting for scientists and researchers. It has been found that most of heavy metals such as Cadmium can eliminate cancer cells in low concentrations. The main mechanism of Cadmium Oxide (CdO) nanoparticles’ effect on cancer cells is through DNA and protein damage and also destruction of the cell wall. In addition to Cadmium, other heavy metals such as Copper and Cobalt have anti–cancer properties. The interesting fact about the anti–cancer properties of Cadmium Oxide (CdO) nanoparticles is that they are not dangerous for human and mammalian cells. As a result of such unique characteristics, Cadmium Oxide (CdO) nanoparticles have been widely used in industrial countries. The action mechanism of Cadmium Oxide (CdO) nanoparticles is similar to other nanoparticles but its main activation is through destruction of the cell wall. This feature of Cadmium Oxide (CdO) nanoparticles makes them very useful for eliminating cancer cells .208-213
On the other hand, Cadmium Oxide (CdO) nanoparticles have opened new horizons to scientists and researchers for the prevention of cancer and its treatment. In this regard, Cadmium Oxide (CdO) nanoparticles–based therapy has emerged as a new branch of nano–based treatments. The Cadmium Oxide (CdO) nanoparticles, which are made in various forms, are widely interested in the field of medicinal and pharmaceutical researches, especially the researches about its applications on cancer treatment. Cadmium Oxide (CdO) nanoparticles are used in various fields such as delivery of drug to the tumor cells, pulling out the cancer of live cells, attacking to cancer cells, improving the sensitivity of cancer cells to imaging and observing them more accurately .214-218
Recently, multifunctional Cadmium Oxide (CdO) nanoparticles have been used for early diagnosis of pancreatic cancer. If pancreatic cancer detects at advanced stages, surgery and chemotherapy usually are not useful. As Cadmium Oxide (CdO) nanoparticles stick to cancer cells, they can easily detect observe by MRI. By testing mice that had been implanted cancerous tumor inside their body, it was found that these nanoparticles which have colorful spectrum near IR region can be observed using especial cameras. This method proves early diagnosis of cancer using Cadmium Oxide (CdO) nanoparticles and supports new hope in finding a cure for cancer. These nanoparticles can be used to identify the precise location of the tumor before surgery, to detect risk margin around the tumor and to track responses to treatment after surgery .219-223
Cadmium Oxide (CdO) nanoparticles are also used for drug delivery to cancer cells. However, due to the very tiny size of the Cadmium Oxide (CdO) nanoparticles, each can carry only small amounts of the drug. Therefore, it is necessary to utilize millions or even billions of these nanoparticles for drug delivery to the exact cancer location. In this way, difficulty to deliver anti–cancer drug to the desired location will be resolved without affecting healthy cells. Recently, successful application of Cadmium Oxide (CdO) nanoparticles in the early detection of cancer cells has been shown. Furthermore, a sensor has been made by Cadmium Oxide (CdO) nanoparticles which can identify lung cancer through patient breath. At the moment, the accuracy of the device which has been tested on a group of healthy individuals and individuals with cancer is 86 percent .224-230
In the current study, for the first time, we originally study linear and non–linear optical characteristics such as fluorescence emission, second harmonic generation and two photons emissions and also medicinal and pharmaceutical applications of Cadmium Oxide (CdO) nanoparticles for eliminating cancer cells. In this regard, the effect of Cadmium Oxide (CdO) nanoparticles on DNA of human cancer cells and the interaction of Cadmium Oxide (CdO) nanoparticles with DNA of cancer cells are investigated.
Non–doped Cadmium Oxide (CdO) films were produced on glass substrate using 1 molar Cadmium acetate solution with distilled water and ethanol. The volume of solution is 65 (ml) and the ratio of ethanol to water is 2:2. The temperature of solution was changed between 450 and 600 ºC. The obtained solution was sprayed with flow rate of 55 (lit/min). By reaching the close drops to the bottom of warm layer, pyrolytic process produces and Cadmium Oxide (CdO) film with high adhesion occurs.
After completion of deposition process, the temperatures of films were reduced to the room temperature. The X–Ray Diffraction (XRD) spectrum of compounds is achieved by PANalytical–X’Pert Pro MPD with Cr Kα rays in the angle range of 2θ = 5°– 80°. The device is used to investigate structural characteristics of samples. ATR–FTIR spectrum in the range of 400–1000 nanometers is obtained by a ATR–FTIR Bruker Spectrophotometer. The spectrum shows optical characteristics of samples. Also, in the current research, grain sizes are calculated by X`Pert HighScore Plus software based on Scherrer equation.
Cadmium Oxide (CdO) nanoparticles were produced by laser ablation of Cadmium plate (99.99%) in distilled water. Before testing, Cadmium plate and beaker were washed and cleaned by alcohol, acetone and distilled water in ultrasonic device (ultrasonic bath). Then, Cadmium plate was placed in a beaker filled by 40 (ml) distilled water and was subjected to laser with 9 (ns) pulse width and repeating rate of 15 (Hz) for 9 minutes. In this test, Nd:YAG laser with switch Q was used. In this state, laser emission with 3–3.5 (mm) diameter was focused using a lens with 95 (mm) focal distance. Cadmium Oxide (CdO) nanoparticles were produced using Nd:YAG laser with 1256 (nm) wavelength and 2-3.5 (J) pulse energy and Nd:YAG laser with 784 (nm) wavelength (second harmonic) and 0.71 and 0.89 (J) pulse energy. Table 1 lists the details of prepared samples. Various detecting analyses were used to identify the characteristics of Cadmium Oxide (CdO) nanoparticles. To evaluate the shape and size of the produced nanoparticles, Transmission Electron Microscope (TEM HD–2700) were used. In addition, size distribution of Cadmium Oxide (CdO) nanoparticles was measured by Dynamic Light Scattering (DLS) system Malvern Zetasizer with particle size range 0.3 (nm) to 10 (µm), temperature range 90 ºC, size measurement of sizes < 1nm, size measurement of molecules with MW < 1000Da and Low volume requirement (as little as 2 (µL)). Variations of electromagnetic absorption spectrum in the range of 400 to 1300 nanometers were measured by PG instruments Ltd spectrometer. The Varian Cary Eclipse Fluorescence Spectrophotometer, equipped with Xenon lamp, was used to evaluate characteristics of fluorescence.
Laser Wavelength (nm) |
784 |
1256 |
||||
Energy of Pulse (J) |
0.71 |
0.89 |
2 |
2.5 |
3 |
3.5 |
Sample |
1 |
2 |
3 |
4 |
5 |
6 |
Table 1 Laser wavelength and pulse energy for prepared samples
Structural characteristics
The structural characteristics of films are studied using X–Ray Diffraction (XRD). Figure 2 shows X–Ray Diffraction (XRD) of the produced films in various temperatures. The results obtained from X–Ray Diffraction (XRD) analysis shows that the produced films are polycrystalline and have a wurtzite structure. By increasing the temperature up to 500º C, a better crystalline structure with a certain preferred direction is obtained. At higher temperatures, the crystalline structure collapses and the intensity of (002) peak reduces. The crystallite size of Cadmium Oxide (CdO) can be calculated by Scherrer equation.
The variations of crystallite size as a function of substrate temperature is shown in Figure 3. Firstly, the crystallite size increases up to the maximum value of 63.7 (nm), at 500º C, with increase in temperature of substrate and then decreases. Increase in crystallite size with increase in temperature may be due to improve in process of reaction between sprayed drops as well as improve in mobility of atoms in the surface of substrate.
Optical characteristics
Figure 4 shows the spectrum transmitted through Cadmium Oxide (CdO) films in a region with wavelength ranged between 400-1000 (nm) at the temperature between 450-600º C. Temperature variation is of a key role in transparency and optical characteristics of films. The diagrams obtained from spectrum transmitted through films are transparent in visible region and by increasing the temperature of substrates, transparency reduces. It can be concluded that increase in temperature makes devastating effects due to penetration of substrate compounds into films and hence, leads to reduce in transparency.
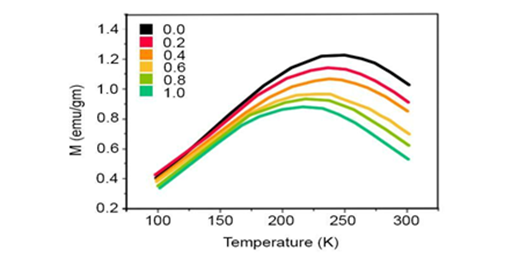
Figure 4 The spectrum transmitted through Cadmium Oxide (CdO) films produced at various temperatures.
Optical, Medicinal and Pharmaceutical Characteristics of Cadmium Oxide (CdO) Nanoparticles
Sample preparation: Cadmium Oxide (CdO) nanoparticles used in the current study are produced by gas evaporation technique. As the goal of the current study is using Cadmium Oxide (CdO) in bioapplications, 0.1 (gr) of the nanoparticles are solved in 55 (ml) of deionized water. All presented results are obtained in room temperature. Figure 1 is pointed out in abstract shows the results obtained from Scanning Electron Microscope (SEM) technique on Cadmium Oxide (CdO) nanoparticles with 50000x zoom. The size of these nanoparticles are about 250-400 nanometers.
Fluorescence: Fluorescence is a spontaneous emission induced by linear stimulation of photons. Fluorescence of semiconductors such as Cadmium Oxide (CdO) can be explained using energy levels. Using a photon with higher energy level than gap energy, it can be possible to stimulate electrons of conducting band and move them to conducting band and hence, only holes with positive charge remain on the valence band. This electron and hole is called exciton. Electrons on conducting band and before recombination can be non–optically transited (usually vibration transition type) between the available compressed levels in conducting band. In this state, the energy difference between electron and hole is lower than the initial state and hence, the produced photon from recombination of electron and hole is of longer wavelength than the initial stimulator photon. If the energy level of stimulator photon is higher than the gap energy of semiconductor, electron moves to higher levels of conducting band but if the energy level of photon is lower than the gap energy, there is no absorption and hence, there is no emission. The single photon fluorescence stimulation of these nanoparticles obtained from stimulation gap and 9 nanometers disclosure with wavelength of 430 and 470 nanometers are shown in Figure 5. As can be seen in this figure, the fluorescence emission of these nanoparticles has a peak in 380 nanometers wavelength (4.4 eV) which is related to gap edge exciton transition.
The other peak, which is wider and is seen in green zone, is induced by surficial deficiencies such as impurities, Oxygen and or Cadmium vacuum. In these nanoparticles, such impurities and hence, the associated transitions were inevitable. However, the emissions induced by deficiencies are not always inappropriate; they can be used as efficient source of white light.
Second harmonic generation
Since Cadmium Oxide (CdO) has a considerable non–linear coefficient, the other part of the current study is focused on investigating non–linear characteristics of Cadmium Oxide (CdO). High power pulse laser with high peak power should be used to observe non–linear characteristics. Tunable Titanium sapphire laser with pulselength of 185 femtoseconds was used to stimulate Cadmium Oxide (CdO) nanoparticles. Cadmium Oxide (CdO) nanoparticles sample solved in water was subjected to NIR emission in quartz cell. Laser light was focused on quartz cell using a lens with focal distance of 22 (cm) and second harmonic was generated. To detect the second harmonic signal, two lenses with wide mount and with degree of 180 from descending light were used. Before entering signal to detector, a NIR filter was installed on the mount of camera to remove the descending laser light. Figure 6 shows the results of second harmonic stimulation of Cadmium Oxide (CdO) nanoparticles in photon counting state and in wavelengths of 800, 825, 850, 875, 900, 925, 950, 975 and 1000 (nm). By distancing from 800 nanometers wavelength, laser power has a descending trend which causes to reduce in second harmonic peaks of these nanoparticles. Figure 6 shows normalized results.

Figure 6 Second harmonic spectrum of Cadmium Oxide (CdO) nanoparticles with various stimulated wavelength: (a) 800 (nm); (b) 825 (nm); (c) 850 (nm); (d) 875 (nm); (e) 900 (nm); (f) 925 (nm); (g) 950 (nm); (h) 975 (nm); (i) 1000 (nm).
Two photons absorption
When Cadmium Oxide (CdO) nanoparticles stimulate with a wavelength that the energy of descending photon (ex) satisfies 4ex>Eg and there is a considerable two photons absorption cross section, two photons absorption occurs. Therefore, if Cadmium Oxide (CdO) nanoparticles stimulate with wavelength ranged between 900–950 nanometers, two photons emissions occurs. In a narrow range (950-1000 nanometers), two photons absorption occurs in addition to second harmonic generation, simultaneously. Figure 7 shows this state for 4 different wavelengths schematically.
As can be seen, emission induced by two photons absorption is wider than second harmonic generation. In this narrow zone, shorter wavelength means that the contribution of two photons absorption is higher and by increasing wavelength, two photons emissions disappears. In Figure 7, Cadmium Oxide (CdO) nanoparticles are stimulated by wavelength between 900 and 930 nanometers and second harmonic generation and two photons emission are simultaneously appeared (in this case, arrangement is similar to second harmonic detection in counting photon case).
Applications of Cadmium Oxide (CdO) nanoparticles in medicine and pharmaceutics
Currently, nano–atto second pulse lasers are used to image from biosamples. Such systems are of very high power peak which is higher than the failure threshold of biosamples. However, average power of lasers is important for non–linear imaging of biosamples. It may be possible that pulse lasers have average power less than 1.5 (W) and peak power more than 1.5 (GW). Since relaxation time between pulses are adequate (repeat rate of about 105MHz), the average power of laser is lower than failure threshold of biosamples while their peak power is of required capability to non–linearly stimulate samples.
Second harmonic generation is only a frequency conversion process and no absorption occurs in this process, so it can be used to image from bioamples. Cadmium Oxide (CdO) is able to pass through cell wall and enters into the cell. In this manner, non–linear characteristics of Cadmium Oxide (CdO) nanoparticles were used for imaging and tracing of animal cells.
The current investigation shows that second harmonic signal of these nanoparticles can be used for stable and longtime imaging and tracing. Since two photons absorption is of more penetration depth and is accompanied by heat production, two photons imaging was always accompanied by heat and sample burning and can be used to eliminate cancer cells.
Figure 8 shows absorption spectrum of Cadmium Oxide (CdO) nanoparticles considering the absorption of distilled water as reference.
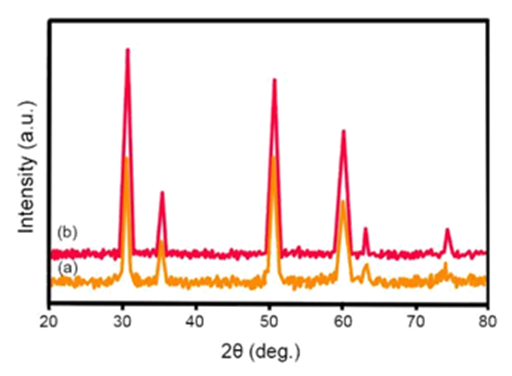
Figure 8 Absorption spectrum of Cadmium Oxide (CdO) nanoparticles in distilled water produced at (a) 784 nanometers wavelength, (b) 1046 nanometers wavelength.
The peak of UV absorption resulted from absorption of Cadmium Oxide (CdO) nanoparticles exciton is happened at 322–363 nanometers wavelength. The effect of size of nanoparticles on electronic structure of semiconductors explains by increase in band gap with decrease in size of particles which attributed to quantum surrounding effect. A blue shift in the edge of absorption was observed with increase in laser pulse energy which can be used to qualitatively describe the size distribution of particles. In smaller nanoparticles (in higher laser energies), a sharp peak of exciton is presented in the absorption spectrum which shows limited size distribution of nanoparticles in the sample and for larger particles, this is not observed in absorption spectrum (in lower laser energies). The reason is the fact that a number of exciton peaks in various energies are related to nanoparticles with various sizes which overlaps each other. Therefore, it can be expected that size distribution of nanoparticles extends. This assumption is confirmed using size distribution of nanoparticles resulted from Dynamic Light Scattering (DLS) analysis in various samples (Figure 9).
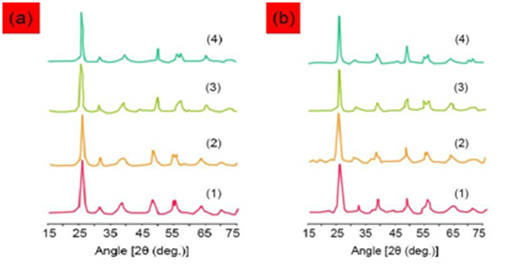
Figure 9 Size distribution of nanoparticles resulted from Dynamic Light Scattering (DLS) analysis (1) sample 3, (2) sample 4, (3) sample 5 and (4) sample 6.
As can be seen in Figure 9, size of nanoparticles is decreased with increase in laser pulse energy. The average size of nanoparticles is listed in Table 2.
Sample |
|||||
1 |
2 |
3 |
4 |
5 |
6 |
Size (nm) to DSL |
|||||
– |
- |
84 |
73 |
69 |
65 |
Size (nm) to TEM |
|||||
83.4 |
51.6 |
87.7 |
68.9 |
45.4 |
37.9 |
Table 2 Size of Cadmium Oxide (CdO) nanoparticles
Considering the fact that Dynamic Light Scattering (DLS) analysis shows hydrodynamic size of particles, the results of this analysis is considerably higher than those resulted from Transmission Electron Microscope (TEM) images. It may be due to the forming of Hydrogen bond between carboxyl group on the next surface, which can lead to transversal connection between particles and hence, larger particles. Transmission Electron Microscope (TEM) images of Cadmium Oxide (CdO) nanoparticles, which are in the scale of about 250 nanometers, are shown in Figure 10. As can be seen, nanoparticles are approximately spherical and their sizes are reduced by increase in laser pulse energy.
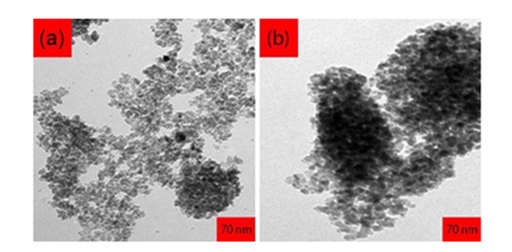
Figure 10 Transmission Electron Microscope (TEM) images of Cadmium Oxide (CdO) nanoparticles with 35000x zoom
Variations of size distribution of nanoparticles by increase in laser pulse energy are similar to the results obtained from Dynamic Light Scattering (DLS) analysis. In other words, size distribution of nanoparticles is uniform. By increasing the laser pulse energy, larger nanoparticles can be simply broken into smaller parts due to interaction with intense laser pulse.
Figure 11 shows the fluorescence spectrum of Cadmium Oxide (CdO) nanoparticles which are stimulated by Xenon lamp in wavelength of 407 nanometers. Usually, emission band in UV and visible fields are observed in fluorescence spectrum of Cadmium Oxide (CdO) nanoparticles. UV peak, which is usually considered as an indication of Cadmium Oxide (CdO) emission, is called edge band emission or exciton transmission. However, emission bands in visible zone are induced by recombination of holes resulted from photon emission with charged, ionized state in inherent deficiencies such as Oxygen blank space, inter–lattice Cd and or impurities. First of all, if stimulation energy is considered considerably lower than gap energy and the second, if the intensity of visible light emission induced by increase in density of deficiencies is very high. In all samples of Cadmium Oxide (CdO) nanoparticles, the peak of UV in fluorescence spectrum is dominant and its intensity increases with increase in laser pulse energy while the intensity of the presented peaks in visible zone is decreased. In other words, severe exciton emission shows that the produced Cadmium Oxide (CdO) nanoparticles are of little deficiencies.

Figure 11 Fluorescence spectrum of Cadmium Oxide (CdO) nanoparticles produced at (a) 784 nanometers wavelength, (b) 1256 nanometers wavelength
General outline of energy level graph of Cadmium Oxide (CdO) nanoparticles is shown in Figure 12. Various deficiencies are considered in this model. The first principle shows that Cd3d electron is severely interacted with O2p electron of Cadmium Oxide (CdO). As can be seen in Figure 12, fluorescence in violet zone can be attributed to transmission from conducting band to deep holes tra pped in a level such as VCd. Blue emission can be considered as direct recombination of conducting electron in Cd3d band and hole in O2p valence band. The usual process for green emission is as following: transmission mechanism (a) from proximity of conducting band edge to deep level of receptor and (b) from deep level of donor to valence band. Recombination of surficial electron trapped in a deep hole trapped in center of V0++ leads to visible emission.
It should be noted that the effect of Cadmium Oxide (CdO) nanoparticles on DNA of human cancer cells is listed in Table 3. In addition, Figures 13 &14 demonstrate fluorescence and photoluminescence of Cadmium Oxide (CdO) nanoparticles in violet zone (a) from proximity of conducting band edge to deep level of receptor and (b) from deep level of donor to valence band, respectively. Moreover, as can be seen, Figures 15 & 16 display intensity dependence of fluorescence and photoluminescence of Cadmium Oxide (CdO) nanoparticles in different wavelengths. Furthermore, Scanning Electron Microscope (SEM) images and also Transmission Electron Microscope (TEM) images of Cadmium Oxide (CdO) nanoparticles (a) before interaction with DNA of cancer cells and (b) after interaction with DNA of cancer cells is shown in Figures 17 & 18, respectively. Also, as another novel achievement, simulation of Cadmium Oxide (CdO) nanoparticles before and after interaction with DNA of cancer cells is illustrated in Figure 19.
Concentrations |
DNA of Human Cancer Cells (ppm) before Interaction with CdO |
DNA of Human Cancer Cells (ppm) after Interaction with CdO |
Control |
1.84±0.23 |
7±0.63 |
0.001 μg/ml CdO |
17.34±0.73 |
1.94±0.01 |
0.003 μg/ml CdO |
1.67±0.42 |
0.01±0.0001 |
0.005 μg/ml CdO |
1.73±0.19 |
0.01±0.0001 |
0.007 μg/ml CdO |
1.94±0.54 |
0.01±0.0001 |
0.07 μg/ml CdO |
2.28±0.11 |
0.01±0.0001 |
0.7 μg/ml CdO |
2.73±0.81 |
0.01±0.0001 |
Table 3 Effect of Cadmium Oxide (CdO) nanoparticles on DNA of human cancer cells
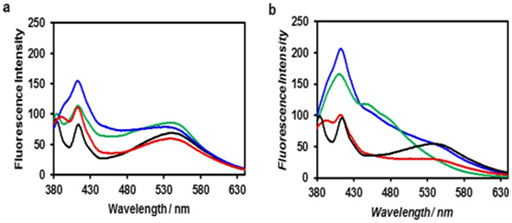
Figure 13 Fluorescence of Cadmium Oxide (CdO) nanoparticles in violet zone.
(a) from proximity of conducting band edge to deep level of receptor,
(b) from deep level of donor to valence band
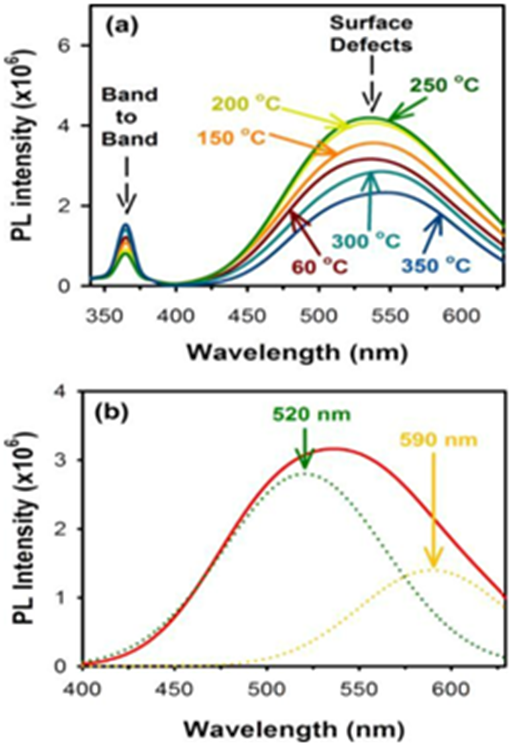
Figure 14 Photoluminescence of Cadmium Oxide (CdO) nanoparticles in violet zone.
(a) from proximity of conducting band edge to deep level of receptor,
(b) from deep level of donor to valence band
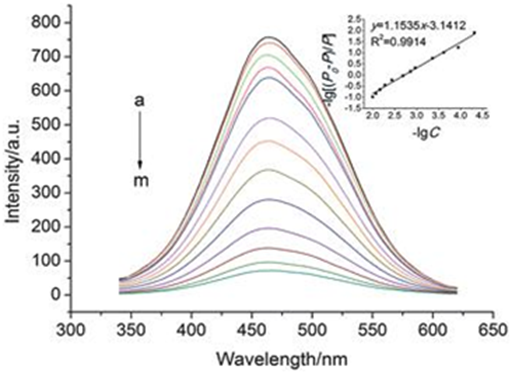
Figure 15 Intensity dependence of fluorescence of Cadmium Oxide (CdO) nanoparticles in different wavelengths.
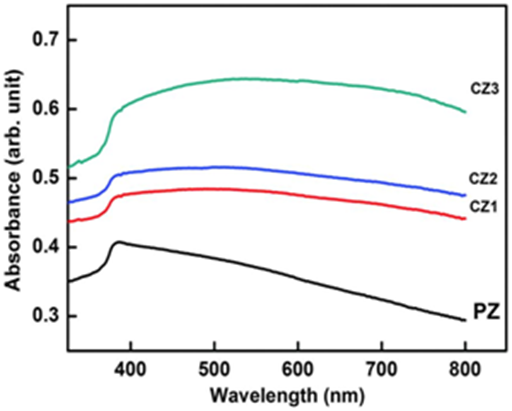
Figure 16 Intensity dependence of photoluminescence of Cadmium Oxide (CdO) nanoparticles in different wavelengths.
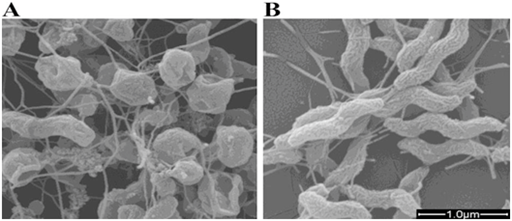
Figure 17 Scanning Electron Microscope (SEM) images of Cadmium Oxide (CdO) nanoparticles with 95000x zoom .
(a) before interaction with DNA of cancer cells,
(b) after interaction with DNA of cancer cells.
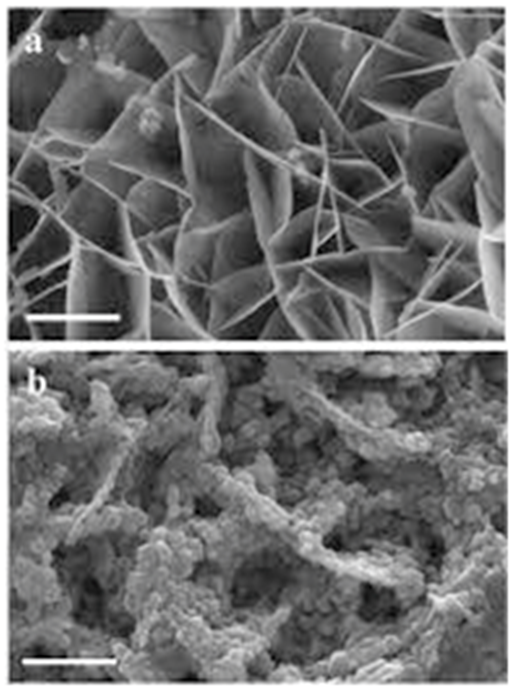
Figure 18 Transmission Electron Microscope (TEM) images of Cadmium Oxide (CdO) nanoparticles with 55000x zoom .
(a) before interaction with DNA of cancer cells,
(b) after interaction with DNA of cancer cells.
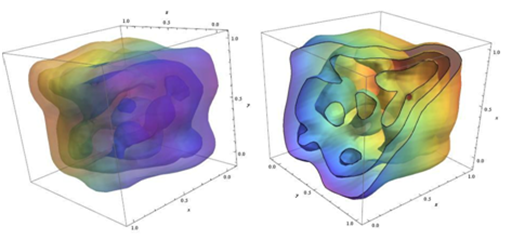
Figure 19 Simulation of Cadmium Oxide (CdO) nanoparticles before (left illustration) and after (right illustration) interaction with DNA of cancer cells.
None.
None.

©2015 Heidari, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.