Journal of
eISSN: 2377-4282


Research Article Volume 4 Issue 1
1UMR Qualitrop, Universite de Guyane, France
2Laboratorio de Nanobiotecnologia Fitofarmaceutica, Universidade Federal do Amapa, Brazil
3Laboratorio de Pesquisa em Farmacos, Universidade Federal do Amapa, Brazil
44 Laboratorio de Absorçao Atomica e Bioprospecca, Universidade Federal do Amapa, Brazil
Correspondence: Caio Pinho Fernandes, Laboratorio de Nanobiotecnologia Fitofarmaceutica- Colegiado de Farmacia, Universidade Federal do Amapa, Macapa, AP, Brazil, Tel 055(96)4009-2927
Received: May 12, 2016 | Published: August 1, 2016
Citation: Leal JDS, Duarte JL, Vilhena JCE, Florentino AC, Bereau D, et al. (2016) Pequiá-Based Nanoemulsion Highlights an Important Amazon Fruit (Caryocar villosum (Aubl.) Pers.). J Nanomed Res 4(1): 00079. DOI: 10.15406/jnmr.2016.04.00079
The fruits from pequiá (Caryocar villosum) are an important source of antioxidant substances. However, despite the great potential of this natural product for pharmaceutical and food industries, to our knowledge, no study was carried out in order to obtain novel nanoformulation with this raw material. Our results suggested that required Hydrophyle-Lipophile Balance (HLB) of pequiá oil is around 12.0 and nanoemulsion with low mean droplet size (191.3 ± 0.8 nm) and Polydispersity Index (0.290 ± 0.040) was obtained. Thus, our studies contribute to valorization of an Amazon fruit, providing for the first time valuable information about nanoemulsion formation using pequiá fruits oil.
Keywords:Fruits, HLB, Natural oil, Non-ionic surfactants
The genus Caryocar belongs to the family Caryocaracea and comprehends 16 arboreous species. Their fruits are used as food and medicine, being also considered potentially useful for cosmetic industry.1. Caryocar villosum (Aubl.) Pers. is widely distributed in the North region of Brazil, being commonly known as pequiá.2 piqui.3 or piquiá.4 Fruits from C. villosum are very characteristic and used as raw material for extraction of yellow oil that is used in culinary and has great biological potential. Several substances were identified on fruits of C. villosum, including oleic acid, steroids and volatile substances, such as β-bisabolene and (E)-nerolidol.5 Several antioxidants substances were also reported for this fruits, including phenolic compounds and carotenoids.3-7 They scavenge reactive oxygen and nitrogen species and therefore may be useful to promote human health.4-7 Moreover, piquiá fruits developed a main role in gene expressions that are related to oxidative stress and generation of free radicals.8 More polar substances such as saponins were also found in fruits.9 and stem barks.10 of C. villosum.
Nanotechnology is a growing multidisciplinary area that is considered promising for development of novel products for pharmaceutical industry, including nanoemulsions.11 Nanoemulsions are dispersed systems constituted by two immiscible liquids and are often stabilized by surfactants.12,13 presenting droplets with mean diameter between 30-300 nm.14 The small size of these droplets is associated to kinetic stability of nanoemulsions during storage, preventing gravitational separation or particle aggregation. They also have a typical translucent or transparent aspect with bluish reflect .15-17 They are very promising to enhance water solubility of vegetal oils and improve chemical stability and bioavailability of bioactive substances. On this context, several studies aiming to obtain nanoemulsions were carried out with important oils from natural origin.18-23 However, despite great biological potential of Caryocar villosum fruits, to our knowledge, no study was carried out aiming to obtain nanoemulsions using its oil. Therefore, the present study presents a novel pequiá oil-based nanoemulsion with great potential for cosmetics, nutraceuticals and phytopharmaceuticals.
Chemicals
Surfactants were obtained from Praid (SP, Brazil) and solvent (analytical grade) were obtained from Vetec (RJ, Brazil).
Extraction of C. villosum fruits
Fresh pulp (30 g) of C. villosum was extracted with 150 ml of hexane using a Soxhlet apparatus during 4 hours. After this period, solvent was removed under reduced pressure using a rotary evaporator with water bath (40 oC). Pequiá extract was stored under controlled temperature (4 oC) and protected from light.
Emulsification method
The preparation of C. villosum emulsions was carried out according to a previous described method.21 The oily phase was composed by C. villosum extract and surfactants (1:1) and was heated at 65 ± 5 °C. Aqueous phase, constituted by distilled water was individually heated to the same temperature of oily phase. Then, water was titrated through water phase using a burette under constant magnetic stirring (800 rpm), obtaining a primary emulsion. Further homogenization step was carried out using a high speed homogenizer Ultra-turrax T25 IKA during 5 min at 8000 rpm for droplet size reduction.
Determination of required Hydrophile-Lipophile Balance (RHLB) Of C. villosum extract
Series of emulsions was prepared by ranging sorbitan monooleate: polysorbate 80 ratios. Therefore, different HLB values were achieved from 4.3 (100% of sorbitan monooleate) to 15.0 (100% of polysorbate 80). All emulsions were prepared as follows: 5% of pequiá extract (w/w), 5% of surfactant (s) (w/w) and 90% of water (w/w).
Characterization of droplet size distribution
Emulsions were characterized by dynamic light scattering using a Zetasizer-nano (Malvern Instruments, UK). They were diluted using deionized water (1:25). Droplet size and Polydispersity Index are represented as mean ± standard deviation in Figure 1.
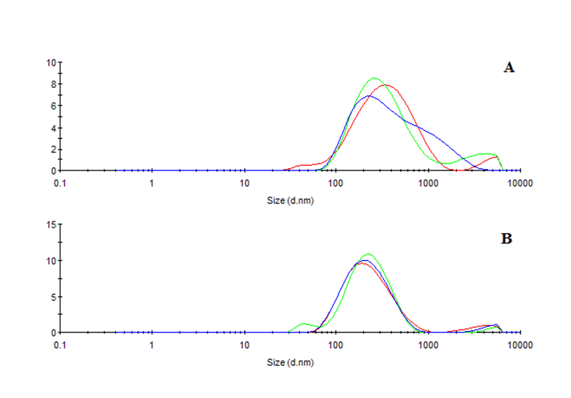
Figure 1 A: Day 1-mean droplet size: 276.6 ± 3.8 / pdi: 0.398 ± 0.000; B: Day 7-mean droplet size: 191.3 ± 0.8 / pdi: 0.290 ± 0.040.
Statistical analysis
The significance of the results was analyzed using One-way Anova with 95% of confidence interval) and Tukey´s test, using Software R (R Core Team, 2013). Differences among mean droplet diameters were considered significant when p < 0.05.
Table 1 shows mean droplet size and Polydispersity Index of emulsions prepared with pequiá extract at different HLB values. Most of them presented instable behavior, such as creaming and phase separation (HLB 4.3-10 and 13-15). Among this first set of emulsions, two of them (HLB 11 and 12) presented a remarkable fine aspect with bluish reflect, characteristic for nanoemulsions. Analysis of mean droplet size after 1 day of preparation confirmed sub-micrometer diameter (HLB 11: 239.7 ± 4, 7 and HLB 12: 276.6± 3.8), however, relative broad distribution was confirmed by Polydispersity Index (HLB 11: 0.438 ± 0.011; EHL 12: 0.398 ± 0.000). Additional emulsion within this range was prepared in order to refine the HLB value determination (10.75; 11.25; 11.5; 11.75 and 12.25). After 1 day, emulsions at HLB 10.75 and 11.5 presented mean droplet size above 300 nm and emulsions at HLB 11.25, 11.75 and 12.25 presented mean droplet size below 300 nm, being considered nanoemulsions. After 1 day of preparation, lowest mean droplet size was observed for nanoemulsion at HLB 12.25, considering all prepared emulsions (198.7 ± 3.729 nm), in addition to low Polydispersity Index. After 7 days, most of them had an augmentation in droplet size, which is not expected for high stable systems.24 During storage, increase on droplet size around 30 to 85% was observed for carotenoid-rich emulsions.25 This class of secondary metabolite is predominant on C. villosum and several carotenoids were identified on the fruits, such as lutein-like and carotene-like substances, antheraxanthin, zeaxanthin, neoxanthin and others.7 Despite slight change was observed for nanoemulsions prepared with surfactants at HLB 11, higher Polydispersity Index was observed, suggesting a polimodal distribution that is not expected for stable nanoformulation. Lower Polydispersity indices are associated to narrow distribution and polimodal distribution may be an indicative of system instable behavior.26-28 Considering all formulations, the nanoemulsion prepared at HLB 11.0 presented the smallest mean droplet size (191.3 ± 0.8 nm) after the storage (p < 0.001) and low Polydispersity Index. This last parameter reflects homogeneity of particle size distribution and values below 0.400 are associated to more stable systems.26 A study carried out with carotenoid-based nanodispersions and using volatile organic solvent revealed a reduction in droplet size, which was associated to solvent diffusion from internal to external phase.29 Considering the analyzed parameter and the fact that nanoemulsion prepared with surfactants at HLB 11.0 had a tendency after 7 days to achieve a monomodal distribution with mean droplet size below 200 nm, we suggest that this is the required hydrophilic lypophile of pequiá extract used in the present study.
|
HLB |
Day 1 |
Day 7 |
||
|
Mean Droplet (nm ± SD) |
Polydispersity Index |
Mean Droplet (nm ± SD) |
Polydispersity Index |
|
|
10.75 |
307.2 ± 4.3 |
0.711 ± 0.031 |
350.1 ± 22.0 |
0.664 ± 0.129 |
|
11 |
239.7± 4.7 |
0.438 ± 0.011 |
237.6 ± 5.19 |
0.430 ± 0.017 |
|
11.25 |
216.7 ± 1.4 |
0.387 ± 0.025 |
231.3 ± 12.3 |
0.409 ± 0.009 |
|
11.5 |
329.9 ± 6.1 |
0.630 ± 0.009 |
344.1 ± 12.0 |
0.583 ± 0.099 |
|
11.75 |
278.3 ± 3.1 |
0.507 ± 0.099 |
287.9 ± 8.0 |
0.488 ± 0.068 |
|
12.0* |
276.6± 3.8 |
0.398 ± 0.000 |
191.3 ± 0.8 |
0.290 ± 0.040 |
|
12.25 |
198.7 ± 3.7 |
0.285 ± 0.026 |
233.3 ± 2.1 |
0.401 ± 0.002 |
Table 1 Particle size distribution of nanoemulsions prepared with C. villosum oil
*Required Hydrophyle-Lipophile Balance of C. villosum oil
All emulsions were prepared with 5% of pequiá extract (w/w), 5% of surfactant (s) (w/w) and 90% of water (w/w).
HLB was primarily defined as a semi-empirical scale for selecting surfactants according to its hydrophilicity or lipophilicity.30 Further studies were carried out by preparing a set of emulsions using a wide range of surfactant blend. Since each surfactant mixture has a typical HLB value, the concept of rHLB of oil could be stated. Among the set of emulsions prepared with surfactant (s) at different HLB values, the rHLB of oil can be determined considering that it should be the HLB of surfactant or surfactant mixture that allowed achievement of most stable emulsion.31 Considering that small droplets, including in the range of nanoemulsions, are intrinsically associated to enhancement of physical stability, it is worth mentioning that rHLB determination is a useful tool to generate nanoemulsions. On this context, several studies with this approach obtained plant-based nanoemulsions using natural oils.18,20-23,32,33
Oils from fruits are valuable source of bioactive compounds. Several of them were subjected to studies aiming to generate novel nanoformulation, such as nanoemulsions. However, studies regarding Amazon species are scare. On the present study, we successfully determined required HLB value of pequiá oil and obtained nanoemulsion with good physical properties. Thus, we provide valuable contribution to nanobiotechnology and valorization of an important species that can be sustainable used to generate novel nanoemulsions for industry.
None.
None.

©2016 Leal, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.