Journal of
eISSN: 2377-4282


Mini Review Volume 4 Issue 2
1Faculty of Mechanical Engineering, University Malaysia Pahang, Malaysia
2Department of Physics and Oxford Martin Programme on Nanotechnology, Oxford University, UK
Correspondence: Sonia Trigueros, Department of Physics and Oxford Martin Programme on Nanotechnology, Oxford University, Parks Road, OX1 3PU, UK
Received: September 30, 2016 | Published: October 29, 2016
Citation: Lah NAC, Samykano M, Trigueros S (2016) Nanoscale Metal Particles as Nanocarriers in Targeted Drug Delivery System. J Nanomed Res 4(2): 00086. DOI: DOI: 10.15406/jnmr.2016.04.00086
In the recent years, noble metal particles such as gold (Au) and silver (Ag) have been used progressively as efficient and safe nanoscale drug carriers in treating malignant as cites of cancerous cells. These single crystal structures of functionalised therapeutic particles with the size less than 100 nm in diameter had proven offered an excellent function to modulate the oxidative stress and toxicity at affected membrane cells particularly to achieve the site-specific delivery of drugs. This mini-review will highlight the current advances of Au and Ag nanoscale particles as smart chemotherapeutic molecule carriers to these malignancies in impeding cancer cell activities locally. The paper also reviewed valuable insights of their efficacy in maintaining and precisely control the drugs release level within the therapeutic windows.
Recent leaps in the field of oncology and therapeutic research of nano diagnostic and nano therapeutic agents has grave importance improvements in the use of nanoscale metal particles as a solid carrier for thesite-specific delivery of drugs release process.1-8 The comfort factors in using nanoscale metal particles are attained from their capability to increase the aqueous solubility of hydrophobic drugs compound, enhanced the circulation time of drugs in the blood, and repress or eliminate fast renal drugs excretion. Nevertheless, the clinical success of these agents is crucially related to the efficacy of the tiny particles to guide the chemotherapeutic drugs to the targeted cell at a designated period of time with in the body. These individual conjugates metal particles dramatically increase the cell-specific drug accumulations.9-12 and opens up the possibility of internally controlled activation (the replacement) of the delivered drug where the therapeutic effect is required in affected cell. This efficacy primarily depends on their physicochemical properties that should fit well in the context as drug conjugates. Therefore, the efficacy, in this case, is defined as maintaining the required distribution of drugs from plasma into a particular tissue or cell, there by preventing possible normal tissues and cells’ damage within or close to the area being treated that might otherwise have. Nonetheless, the real challenge is a bit stiff still. The particles become thwart themselves and tend to halt the binding or producing the drug’s binding incompetency at thespecific receptor, and least of these is not capable of reacting by themselves into the pathways that led down to the drug inducement system andproduced the side effect.13-15 Thus the drug delivery system demanding nanoscale particles should be exploited ina very delicate ways that can improve the capabilities of the therapeutic agent to optimise and implement the system in a safe and precise manner.
Nanosize Ag and Au particles have been used in elixirs and tonics for medicinal application since time immemorial due to their exciting antimicrobial properties to manipulate the fluids to generate monodisperse and uniform droplets in micro size system.16-18 The therapeutic properties of these metal particles have been identified for thousands of years.19,20 Indeed, the look on Ag and Au nanoparticles as the promising carriers become heavily researched area focus to date where the efforts in the implementation of these particles in the oncology and therapeutic fields increasing. The exponential growth in the number of well-research examples dealing with nanoscale Ag and Au particles for diagnostic and theranostic applications comprehensively justifies their significant promises and impact of treatments’ efficacy is shown in (Figure 1). This exciting discovery towards diagnostic and theranostic applications are continuously merge their way into better medical implementation not only as drug delivery ‘transport’, but they themselves are the drugs.
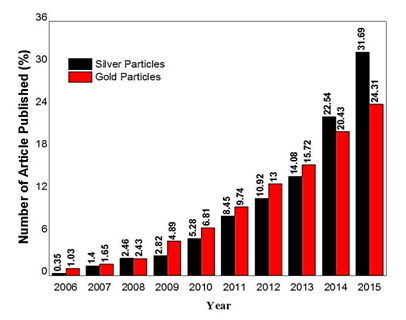
Figure 1 Comparison of temporal evolution in the number of scientific papers published for Ag and Au particles in drug delivery. The total number scientific papers published for Ag particles is lower than Au particles approximately 284 and 2423, respectively, for the past 10 years. (Source: ISI Web of Knowledge with the search terms under ‘drug delivery using Ag and Au nanoparticles’).
The assessment of the apoptotic potential of nanoscale Ag and Au particles with the diameter less than 100 nm have shown such great potential to facilitate site-specific drug delivery.21,22 Importantly, the diameter of the particles should not be more than 100 nm to avoid opsonisation and subsequent elimination of the immune system.23-26 The advantages of using Ag and Au nanoscale particles for drug delivery applicationinclude the ability of these structures to:
Both nanoparticles can be guided and held in desired sites due to their unique electrical and magnetic behaviours by either magnetic or electric fields and the resultant current induced the local heating effect in cancerous regions. This local heating enhances cancerous membrane oxygenation and chemosensitivity and that trigger the release of the loaded drugs or to causes cell death by temperature-induced apoptos is.27 Practically, the implementation of these particles as nanotherapeutic agents is observed as light scattering contrast agents monitored under MRI during photothermal ablation treatment via region-specific magnetic targeting.At the same time, their potential as effective photothermal therapy carriers facilitates their significant endorsement as sufficient absorption antibodies. However, both particles also exhibitseveral drawbacks and challenges;notably, they have greater tendency to aggregate as they encountered with larger or smaller nanoscale particle counter parts, based on the same metal content.28,29 Therefore, a combination with synthetic polymers or also known as surfactantent rapped both particles in organic stimuli-responsive matrices that form well-defined self-assemblies of polymeric nano metal structures. The layers of the adsorbed or covalently bound polymers act as an anti-coagulant,preventing particlesre-aggregation. These polymeric metal particles facilitate the development of secondary functionalised metal particles attached to drugs or molecules that further protect them against recognition of immune system. These interactions are multivalent in nature, and thus the Ag or Au particles mimic the multivalent presentation of therapeutic substances on particles surface to make these functionalised nanoparticles having excellent qualities as good engineered particle carriers.
In particular, nanoscale Ag particles are most frequently synthesised by chemical reduction method where the solutions of Ag precursor salt is chemically reduced in the presence of reducing agent. The system is further stabilised by surfactant which enhances stability (aggregation) and oxidation of the metallic Ag particles as a consequence of microscopic interaction such as the Van der Waals (between molecules and particles) and depletion forces (from excess surfactant).30 The properties of Ag particles for the therapeutic application does control by several factors include the morphology (size and shape), surface chemistry and surface charge as well as the state dispersion of particles.31,32
The functional properties influenced by the size and morphology of Ag particles mainly responsible for the de-localisation of the therapeutic drugs inside the malignancies. In most cases, particles with diameter size less than 120 nm were reported to be well suited for localised drug delivery applications due to the fact that they can be synthesised on a large scale with high monodispersed with almost no drug loss to the malignant sites. This point has previously been made by several authors.33,34 that the localisation behaviour of Ag particles within the malignant areas is dependent on the size as their direction can be sterically hindered in cellular matrix due to the mucoadhesives of encapsulated chemotherapeutic substances.35 The particle size of conjugate Ag particles along with their morphology does indeed give better surface chemistry effect which arises the opsonisation behaviour. The smaller the conjugate Ag particle size, the greater the accumulation inside the malignant sites that in turn shown better circulation and dispersion through different types of membrane cell.36 However, smaller particles can also increase the cytoxicity inside the membrane cells if the adherence and degradation, as well as clearance circulation, are not fully understood although Ag particles modulate both oxidative stress and the cellular uptake efficiently.
Interestingly, surface chemistry role during clearance or uptake in circulation accommodates the facilitation of nanotherapeutic carriers when an abundance of small nanoparticles exist within the malignancies.37 Studies revealed that nanoscale Ag particles had shown a prolonged circulation type of the half-life nanoparticles which can escape from affected cell in the tissue effectively. Long circulation is needed in chemotherapeutic treatment where the drug degradation should be negligible, therefore, undergo apoptosis where the affected cell shrink and produced condensed morphology due to the increased distribution of drug compound.
In most cases, Ag particles are considered as one of the photothermal the rapeuticagents due to their de-localised nature behaviour that forming a sea of the conductive electron which increased thepolarisability of these charge carriers at the surfaces ofAg particles.38,39 (Figure 2). Since Ag particles is neutrally charged particles, their cytoxicity islower than the charged ones.The attractive force between conduction electrons of Ag particles and malignant membrane favour the adhesion rate onto the surface of the targeted cell. Small Ag particles with diameter size less than 100 nm prove to alter the malignant potential effectively as well as impedes its proliferation and induces fluidity of the affected cell sites.
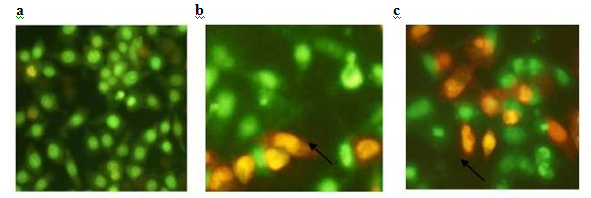
Figure 2 An example of morphological changes caused by nanoscale Ag particle due to the apoptosis. Ag particles treated cell exhibited condensed morphology. (Reproduced from.38).
Au nanoparticles are extremely tiny solid balls made of gold with diameter vary from 5 to 100 nm.40 The Au nanoparticle can be synthesized by various method such as seed-assisted growth.41 wet chemical.42 microwave assisted.43 laser ablation.44 and etc. Wet chemical is the most popular and commonly used method to synthesize Au nanoparticles. In this method the gold salt is reduced in the presence of reducing agent which also acts as stabilising agent. Where else, the capping reagent is used to control the growth and aggregation of the Au nanoparticles. Zeta sizer, transmission electron microscopy (TEM) and/or surface scan electron microscopy (SEM) are some of the methodologies used to characterize and determine the shape, size and size distribution of the synthesized gold nanoparticles.45
To date, a wide variety of sizes, shapes and structures of Au nanoparticles has been reported depending on the application at hand as shown in (Figure 3) Au nanoparticles have been shown as one of the promising and favarouble material in nanomedicine. These Au nanoparticles have been extensively studied and used as therapeutic agents (drug delivery).46 diagnosis agents.47 photothermaltherapy.48 and imaging agents.49 Their tiny size, which meets the dimension of the most biological compounds, high surface area, relatively ease preparation, ease surface functionization make them particularly fascinating towards medical application. However, the interaction of Au nanoparticles with the surrounding biological environment has a significant impact towards their biological activity, as such a methodical understanding of the behaviour and nature of the interactions is essential for proper designing of these nanoparticles for diagnostics and therapeutic applications.50
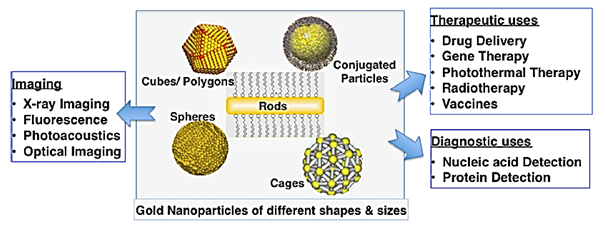
Figure 3 Wide range of potential biomedical application of Au nanoparticles depending on their synthesised size and shape (Reproduced from.40).
There are four main physical and chemical properties to which the Au nanoparticles have been tested shown to be promising candidate for clinical studies and biomedical applications: chemical inertness; surface properties; electronic structure; and optical properties. The high surface area and the relatively small number of ligands coated on smaller diameter particles cause reduced flocculation, where else the larger nanoparticles form insoluble aggregates. Additionally, the chemical inertness of this particle, facilitates gaining them in the wide range of shapes without compromising the high stability, low toxicity and immonugeneity which are essential for biomedical applications.51 Recall, most of the practice employed Au nanoparticles (Figure 4a-4c) have been put as the preferable particle structure as drug delivery vactors in theragnostic field.
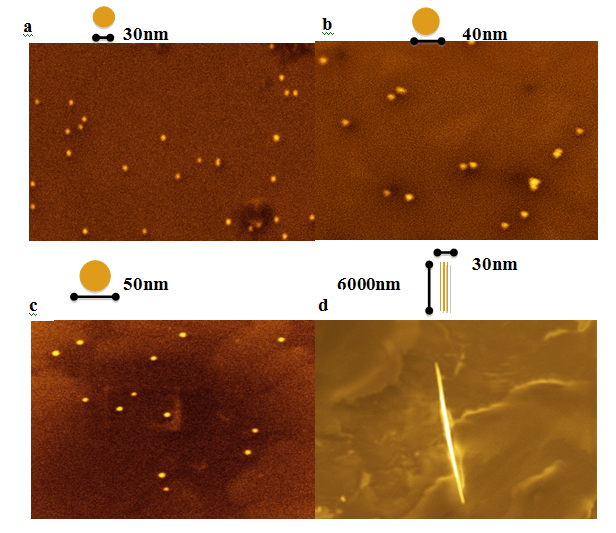
Figure 4 SEM images of Au nanoparticles (a to c) and Au nano wires (d) obtained by seed-assistance and wet chemical methods. (Images provided by Trigueros lab).
Nevertheless,other Au nanostructures such as Au nanorods have also gained much attention due to their remarkable multifunctional properties especially in macrobiological system that needs the drug carriers to be half of the size of DNA molecule. For instance, Au nanorods not only can identify the attendance of tweak genes, but also assists reserchers to precisely spot the affected location of the changes.52 The assemblies of nanorods can simultaneously bind compacted genes and targeting the molceules in the acute way. The precise control of the size and structure of Au nanorods (Figure 4d) allows the efficacy in the chemotherapeutic treatment.
Presently, the controlled delivery of the active biomolecules in live cells or tissues to improve the therapeutic outcomes is one of the major targeted area in biomedicine.52 Nevertheless, the intracellular release of biomolecules in the areas of lession remains a major challenge. This due to the deficiency of physiological solubility of the biomolecules and also the low cell membrane permeability. Thus, higher dose usually preferred and this likely to cause side effects. The Au nanoparticles has been shown to have the potential to overcome this issues and become the ideal material for drug targeting and imaging-based detection. Due to their size smaller than the biological compounds, the active biomolecules can be loaded or attached to the surface of nanoparticles which allows delivering of those molecules directly inside the cells cytoplasm and cell organelles. Interestingly, apart from the size, this particle also exhibits high-density surface allowing high yield ligand anchorage, targeting cellular delivery, controlled intracellular release and ease the transmembrane delivery.52 Specifically, in cancer therapy, the Au nanoparticles based vehicles found to enhance the “in vivo” and “in vitro” therapeutic activity of diverse chemotherapies such as breast cancer stem cells.44 doxorubicin on human glioma cells, human melanoma cell lines.39 temozolomide, and to promote crossing the blood barrier, thereby facilitating greater accumulation of drug in cancer cells.53
The serious implementation of high-throughput Ag and Au nanoparticles in the theragnostic system have devoted improved functionality result to date. While going forward, there are remains of the biggest challenge yet to be solvedin order to perceive the acceptance of these systems as first line treatment modalities. The pressing concern is to systematically deliver both Ag and Au systems in the mass balance for cancer therapies and diagnostics with efficient clearance or excretion with consideration of the subsequent accumulation of every microgram of these therapeutic drugs that are administered. It may also be better to eliminate myth and many common misapprehensions about Ag and Au nanoparticles include their high synthesis cost in amass scale and our relative lack of exposure to these systems throughout much of the 21st century.
None.
None.

©2016 Lah, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.