Journal of
eISSN: 2377-4282


Review Article Volume 2 Issue 3
1Department of Physics and Astronomy, The University of Texas Rio Grande Valley, USA
2Department of Chemistry, The University of Texas Rio Grande Valley, USA
3Department of Biology, The University of Texas Rio Grande Valley, USA
Correspondence: Mircea Chipara, Department of Physics and Astronomy, The University of Texas Rio Grande Valley, 1201 W. University Drive, Edinburg, TX 78539, USA, Tel 1 956 605 5123
Received: June 13, 2015 | Published: September 3, 2015
Citation: Chipara M, Ibrahim E, Yust B, Padilla D, Chipara DM (2015) Nanoparticles and Bacteria. J Nanomed Res 2(3): 00033. DOI: 10.15406/jnmr.2015.02.00033
A concise critical review of the antibacterial features of some nanoparticles (metallic and oxide semiconductors) is presented. The review starts with a brief analysis of the physical features of nanoparticles and includes a discussion about the system nanoparticles-water-light, which is of utmost importance in biological and medical cases. Both metal and semiconducting nanoparticles are releasing electrons under the effect of the incoming electromagnetic radiation (light). This is the source of major misunderstandings as the antibacterial features of nanoparticles can be intrinsic (in this case antibacterial capabilities will be measured in the absence of light) or mediated (in this case the antibacterial features will be observed solely under light irradiation). The critical review opens the discussion about the need for experimental measurements in dark and under the effect of light- a type of research that was typically disregarded. The main ideas are supported by examples from specialty research. New concepts as specificity and effectiveness of antibacterial features are tentatively suggested.
Keywords: Nanoparticles, Bacteria, Photochemistry, Reactive oxygen species, Electromagnetic radiation (light), Mediated antibacterial features, Effectiveness, Specificity
VB, Valence Band; CB, Conduction Band; ROS, Reactive Oxygen Species; NO, Nitric Oxide
Antibacterial features have been reported for various nanoparticles including metallic nanoparticles (such as Ag and Cu) and semiconducting nanoparticles. Most research was focused on semiconducting oxides (such as TiO2, ZnO, and iron oxides). Reports regarding the antibacterial features of metallic and semiconducting nanoparticles are contradictory, revealing the importance of the valence state, the contribution of electromagnetic interactions, and the role of water. In such cases, it is important to disseminate between intrinsic antibacterial properties and facilitated (or mediated) antibacterial features. Intrinsic antibacterial features require a direct and major role for the nanoparticle in the antibacterial process. In the case of facilitated/mediated antibacterial properties, the presence of nanoparticles is required but not sufficient to ignite antibacterial properties. In these cases, the nanoparticles are just components of a more complex (antibacterial) mechanism, which must be activated by other parameters such as electromagnetic radiation, electrochemical processes and/or water.
Finally, the effectiveness and the specificity of the antibacterial features have not been currently investigated. The specificity should quantify the ratio between number of bacteria affected/killed by the nanoparticle and the number of healthy cells affected/killed by the same nanoparticle. Typically, the specificity applies to a pair bacteria-cell. The effectiveness of the nanoparticle as an antibacterial vector is a wide concept that comprises all pair’s bacteria-cells and generates a medical landscape for the use of nanoparticles.
The need for this approach derives from the fact that most studies aiming at the antibacterial features of nanoparticles are quantifying such characteristics based on the effect of nanoparticles on a culture that contains solely the bacteria and eventually some nutrients. It is easy to imagine a scenario where the nanoparticles are killing both good cells and bacteria. This aspect is generally neglected (based on the false premise that if the bacteria are killed, then medical applications are automatically implied).
Infinite crystal versus finite crystal
For a better understanding of the fine modifications caused by the transition from infinite to finite crystals, it is important to focus on the electrons. Figure 1A is represented an ideal 1-dimensional infinite crystal. In this case, the length of the crystal is infinite and the number of repeating motives n, is very large (n→∞). For crystals, these repeating motives (or units) may be represented by the crystalline lattice (primitive cell for example) The electrons are moving within some potential barriers of (identical) height Vb and thickness b, typically represented by the ionic moieties of the crystal. In a metal, these potential barriers are small compared to the kinetic energy of electrons. Accordingly, the electrons move almost freely within the crystal - a situation that is modeled as the free electron gas.
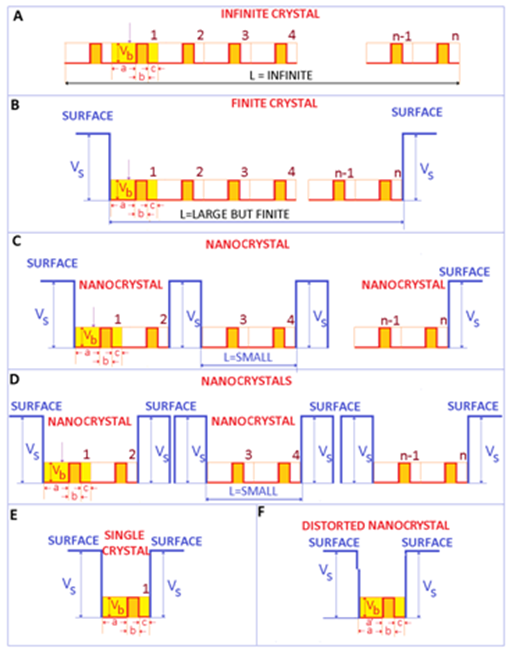
Figure 1 Electronic structure of a finite 1 dimensional crystal, showing the surface contributions, important in the photoelectric effect of metals. A. Ideal infinite crystal. B. Ideal finite crystal. C Ideal nanocrystal. D. Assembles of ideal nanocrystals. E. Single crystal. F. Distorted (real) single crystal (and nanocrystal for few repeating distorted units).
Real crystals have a finite size. Consequently, there is a surface (sometimes named boundary), which limits the extent of the crystal and separates the crystal from the environment or neighboring crystals. This implies that L is finite (although it can and should be very large). If L is very large, it is expected that surface contributions may be negligible compared to volume (bulk) contributions.
For processes that involve the removal of an electron from the crystal (such as the photoelectric effect) as soon as the electron leaves the crystal, the crystal becomes positively charged and starts to attract the departing (negative) electron. This may provide be a qualitative representation of the potential VS. Hence, in the photoelectric effect, certain energy is actually required to extract the electron from a metal. For an infinite crystal, this is shown in Figure 1A. For a finite but large (bulk) crystal this situation is shown in Figure 1B, where the number of repeating structures n is very large, and accordingly L is large although finite (for 1 dimensional metallic materials/crystals). If the size of the material (represented by L) confined between the surface potential VS is in the submicron range, a nanomaterial or nanocrystal is obtained (Figure 1C). The main difference between the bulk crystal and the nanocrystal derives from the small spatial extension of the crystal L, which should be smaller than a critical length ξ - typically of the order of 100 nanometers.1 As the size of the nanocrystal L is reduced below this critical length, the energy levels of electrons are pushed up1-3 (It is important to point that the energy levels are pushed out as the size of confinement is decreased even at larger scales-not solely at the nano scale. However, this effect is stronger at shorter distances). Therefore, when dealing with bulk materials, the surface effects are typically neglected, while for nanomaterials, surface effects typically dominate over volume effects.2
Electromagnetic radiation, and in particular light may be one of the triggering parameters for the electronic processes occurring within metals and semiconductors that are finally responsible for the (chemical) attack on bacteria. The incoming light may provide the required amount of energy to promote the electron into the continuous spectrum (i.e. to free the electron). Visible light extends from about 350 to 750 nm, which in energy units represents about 1.6 to 3.3 eV. Ultraviolet light extends from 3.3 eV up to 102 eV. The photoelectric effect theory defines the energy (work function) necessary to extract an electron from a given target. This energy can be as low as few eV for group 1, alkali metal elements (Na =2.4 eV, K=2.3 eV) or as high as 5 eV for C (diamond). For metals such as Ag and Cu, the average work function is about 4.5 eV and 4.8 eV, respectively. Therefore, the visible light provides sufficient energy to remove electrons from finite crystals made of atoms of group 1 alkali metal elements, while the UV component may be necessary to remove electrons from other elements.
Semiconductors
A semiconducting material has a well-established electronic structure, consisting of two bands separated by an energy gap (Figure 2). In molecules with pi bonds, the molecular energy levels are split into a lower set known as Highest Occupied Molecular Orbitals or HOMO and an upper set known as Lowest Unoccupied Molecular Orbitals, or LUMO. As the number of molecules increases, the number of energy levels in both upper and lower sets increases. For sufficiently large number of molecules, HOMO degenerates into a valence band (VB) while LUMO degenerates into a conduction band (CB).1
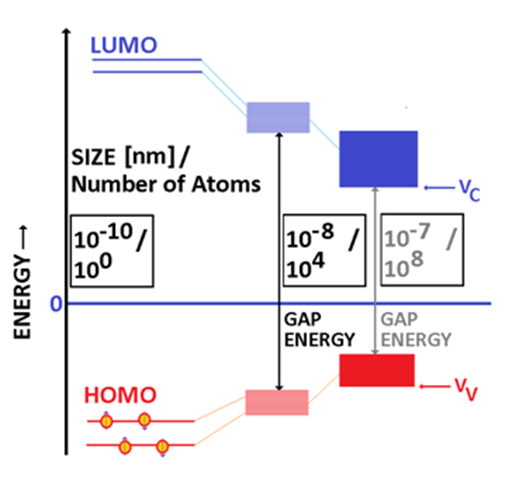
Figure 2 The formation of the energy gap as the size/ number of atoms in the particle are increased. From left to right molecules, nanometer sized semiconducting particle, bulk semicondutor particle. It is noticed that the energy gap is decreasing as the size (number of atoms) that builds up the particle is increased.
In an ideal semiconductor, VB is typically completely filled (at low temperatures). The potential of the top of the valence band is typically labelled as VV the bottom of the conduction band is labelled as VC and the corresponding electronic states are usually completely empty at fairly low temperatures. The energy gap EG is given by EG≥VV-VC≥0. The energy gap can be as low as 0.08 eV in alpha tin or as large as 6.36 eV as in the case of boron nitride, which is frequently classified as a wide band semiconductor. Materials with larger energy gaps are typically classified as insulators. In the general case, the potentials VV and VC depend on the wave vector (momentum) K. If the maxim of the VV and the minim of the VC are occurring at the same value of K, then the gap is direct (and the semiconductor is named direct semiconductor) with EG=VV-VC≥0 (Figure 3).2 If the two extremes are not at the same wave vector then an extra energy is required to promote the electron from the valence to the conduction band. The gap is labelled as indirect gap and the semiconductor is named indirect semiconductor (Figure 3).

Figure 3 The energy gap in semiconductors. Upper panel depicts a direct semiconductor while the lower panel an indirect semiconductor.
Most important direct band semiconductors are: ZnO, TiO2 (rutile), CuO4 and NiO. Among indirect band oxide semiconductors the most known is TiO2 (anatase). Some iron oxides can exhibit both direct and indirect bands.5,6 Details about the spectroscopic features of TiO2 (in dark or irradiated by electromagnetic radiation) are available in many publications.7,8
For clusters and nanoparticles, the picture is complicated by quantum and surface related issues. From the quantum point of view, the electron is localized within a potential well of a given width. As the size of the nanoparticle is decreased, the width of the potential well decreases, and the energy levels are pushed up.3,9 Experimental data indicated that actually the energy gap increases as the particle size is decreased and that the shape of nanoparticles may also affect the value of the energy gap.10 An alternative path to decrease the energy gap implies the doping of the semiconductor. For example, the doping of TiO2 by nitrogen reduced the energy gap and additionally allowed for the photoproduction of *NO.11
By increasing the temperature towards room temperature, the electrons from the valence band may receive sufficient energy to be promoted into the conduction band. However, a temperature of 300 K corresponds to energy of about 26 meV. Typically, this energy is too small to promote the electrons from the VB into CB. Nevertheless, electromagnetic radiation and mainly visible and UV light can excite them from the valence band into the conduction band. While the electron is promoted from the valence band into the conduction band, it leaves within the valence band its image, as a positively charged quasiparticle named hole. Under the effect of an external electric field, the electrons will move towards the positive electrode while the holes will be attracted by the negative electrode.
Metallic nanoparticles
Neutral metallic nanoparticles have a reduced chemical activity and hence are expected to exhibit poor antibacterial features or even no antibacterial properties. Electrically charged metallic species (ions) exhibit chemical reactivity. The general concept is that the metallic nanoparticles that correspond to positive ions are exhibiting antibacterial properties. The generation of positive ions can be achieved either by irradiation with electromagnetic ways (typically in UV) via the photoelectric effect or electrochemically. This suggests that the antibacterial properties of metals (such as Ag, Au) are not active in dark, as the light is required for the formation of the positive ions. However, in the case of metallic iron, a recent paper claims antibacterial features for FeO (on Escherichia Coli).12 The case of nanoparticles is more complex as surface states and surface defects may result in a not negligible concentration of positive ions on a globally neutral or almost neutral nanoparticle. Unfortunately, the dissemination between dark and light-induced antibacterial features of metallic nanoparticles is not a common research objective, yet.
Nanoparticles of semiconducting oxides
Semiconducting oxides such as TiO2, ZnO, FeO, Fe2O3, Fe3O4, NiO…may have frequently an important number of defects and in particular a large number of oxygen vacancies.13 These can affect significantly their electronic features and may add unexpected physical properties. For example, it was speculated that hydroxyl groups may trigger magnetic features in titanium oxides.14 This demonstrates that the interaction between semiconducting oxides and water can result in complex and unexpected consequences. Poor stoichiometry should be carefully assessed,13 especially in nanomaterials where the surface to volume ratio may have an important contribution.13 The ideal nanosized semiconductor has an energy gap, whose value is controlled by size, impurities (or doping), defects, and eventually shape. The actual energy gap determines the minimum frequency of the incoming electromagnetic radiation that can excite the electron into the conduction band and eventually remove it from the semiconductor.
The analogous phenomenon in metallic nanoparticles is represented by the photoelectric effect, which finally ejects an electron from the metallic nanoparticle if the frequency of the electromagnetic radiation exceeds a certain threshold value.
Water, light and semiconducting nanoparticles
Cells, tissues and organs contain an important fraction of water. For this reason the cocktail of semiconducting or conducting nanoparticles and electromagnetic radiation is sufficient to generate free radicals or/and ions capable to interact chemically with cells and to have sizable biological and medical consequences. Reactive oxygen species (ROS) are formed when a semiconducting (metallic) particle, and adequate electromagnetic radiation (and water) are simultaneously present. An adequate electromagnetic radiation is an electromagnetic radiation whose frequency ν is greater or at least equal to the ratio between the energy gap and the Planck’s constant h (ν=EG/h). Such electromagnetic radiation can promote an electron from the valence band into the conduction band leaving a hole within the valence band (Figure 4). In metals, this energy may be sufficient to extract the electron from the metallic nanoparticle. In the case of semiconductors, the energy balance schematically represented by equation (1), is responsible for the generation of electrons and holes:2,15
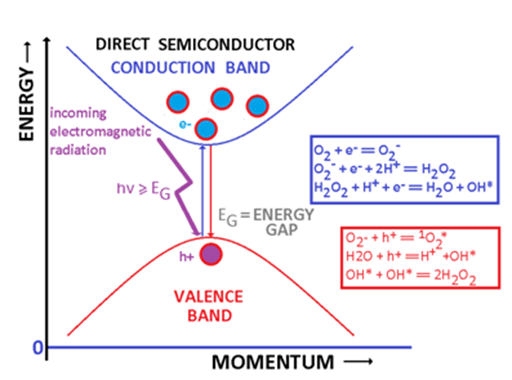
Figure 4 Water, semiconducting/metallic nanoparticles and electromagnetic radiation. A damaging cocktail in biological systems.
Semiconductor + Electromagnetic Radiation (hν≥EG)→h+ + e- (1)
Where the h+ represents the hole (which is positively charged) while the e- is associated to the electron promoted into the conduction band. In most cases, ROS includes oxygen containing free radicals and ions. However, some authors include in ROS free radicals and ions that does not contain oxygen and hydrogen atoms.
Figure 4 represents some potential reactions initiated by the electron released in the conduction band and by the hole. These reactions require either the presence of water (although some reactive species like O2- can be generated just in the presence of oxygen). The most important reactive oxygen species are:16-18
1) Superoxide anion *O2-.
2) Hydroxide radical *HO
3) Hydrogen peroxide H2O2.
4) Ozone, O3
5) Singlet oxygen
Where * identifies a free radical state. Some authors include in ROS the nitric oxide radical *NO, hypochlorite ion OCl-, etc….
ROS identifies highly reactive free radicals and ions, capable of attacking biologic molecules including DNA and proteins. It is generally speculated that ROS have an important role in:19 aging, cancer, and cell evolution (birth, growth, modification, and death).
TiO2 has a complex photochemistry, analyzed in detail by.17,20 The possibility of Ti-OH structures has been confirmed by FTIR spectra.7 The presence of free radicals in TiO2 suspensions exposed to H2O2 and light has been confirmed by electron spin resonance spectroscopy.21 A similar mechanism for the generation of Zn2+ ions in ZnO exposed to H2O2 has been suggested for the explanation of the antibacterial features of ZnO.22 A research on nanofilms of the effect of electromagnetic radiation on TiO2nanofilms in contact with water is described in.23 Recent studies indicated that ROS can be also generated by iron oxides (Fe3O424). The biological effects of ROS are discussed in several articles.25,26 The chain of chemical reactions associated with the oxidation of biological molecules is analogous to the chemical oxidative degradation of polymers, and it is frequently described by the following equations:
Initiation (production of radicals)
RH + O2 → ROOH (preliminary reaction, usually ROOH are labile compounds).
ROOH → *RO + *HO
ROOH + *OH → *ROO + H2O
RH + *OH → *R + H2O
Propagation
*R + O2 → *ROO
Transfer reactions
*ROO + RH → *R + ROOH
*RO + RH → *R + ROH
Termination Reactions
*R + *R → R – R
*RO2 + *R → R-RO2
*RO2 + *RO2 → 2RO2
Consequently, the antibacterial properties may be in some cases a result of the simultaneous presence of 3 factors, oxide semiconducting (or metallic) particles, electromagnetic radiation (of adequate wavelength), and water. In the case of TiO2 nanotubes the outcome of this cocktail is an obvious antibacterial behavior, as confirmed in.27 This manuscript supports the observation that actually the antibacterial features may not be a direct result of the presence of TiO2 but a consequence of ROS interacting with the membrane of bacteria. A better knowledge of the interactions between TiO2 (or ZnO), electromagnetic radiation, and water is mandatory in understanding the chemical processes and risks implied by the use of sunscreens and/or cosmetics based on oxide semiconductors.28 Recent studies suggest that sonication can replace the electromagnetic radiation in the generation of ROS in TiO2.17,29
Potential biological and medical applications
Metallic nanoparticles such as Ag, and Au30 have been known to have beneficial medical applications since ancient Greece. Recent studies indicated that Au and Ag and in particular Ag ions have antifungal properties.31 Unfortunately, the effect of light on the antifugal features has not been investigated. Spectroscopic investigations revealed the formation of a biocomplex between human hemoglobin and silver nanoparticles.32 Later, it was recognized that actually silver and gold have important antibacterial features against both Gramm positive and Gramm negative bacteria, and that these features are enhanced if the particles are well disperse and in colloidal form.30 It was concluded that silver has the best antimicrobial features. Unfortunately, the authors did not disseminate between light and dark antibacterial features and did not try to monitor the eventual production and evolution of ROS species. Gold nanoparticles deposited on zeolites have efficient antibacterial features (Escherichia coli and Salmonella typhi). Unfortunately, dark tests have not been reported.33
It was reported22,34 that nanoparticles of semiconducting oxides are more efficient as antibacterial agents against Gramm positive the Gramm negative strains of bacteria, with stronger antibacterial activities for ZnO (compared to CuO and Fe2O3). The authors speculated that the surface to volume ratio has a sizable contribution on the antibacterial features.34 The study was performed on a system that included nutrients, various bacteria strains, and nanoparticles. The presence/absence of light was not discussed. The effect of nanoparticles’ concentration was not investigated. Nice FTIR and WAXS data on nanoparticles are reported.
While not directly connected to medical applications,35 focuses on the synthesis and photocatalitic activity of TiO2, describing the chemical reactions occurring in TiO2 under the effect of electromagnetic radiation (solar radiation). This is a good starting point in the analysis of the use of semiconducting oxides (TiO2 and ZnO) as major components of sunscreens. In order to understand the role of TiO2 nanoparticles in biological system we have to start from TiO2 nanoparticles and water. This is exactly the subject of the research reported in.36
The production of ROS by semiconducting/conducting nanoparticle (such as TiO2) and light in cell cultures (human cervical carcinoma cells HeLa) has been confirmed.11 A study aiming directly at TiO2 exposed to H2O2 in ambient light and dark demonstrated that free oxygen containing radicals (ROS) could be generated in the absence of light if H2O2 is present. The formation of hydroperoxyl and hydroxyl free radicals was confirmed by electron spin resonance spectroscopy.
An excellent example about the complex biological consequences of the cocktail water, semiconducting nanoparticles, and light is provided by the demonstration that TiO2 nanoparticles are phototoxic to marine phytoplankton.37 This remarkable study includes TiO2 nanoparticles, water, and electromagnetic radiation. It is revealed that UV radiation is required to turn on the antibacterial capabilities of TiO2.
Nanoparticles of TiO2 are frequently used in cosmetics either as white pigment or for UV protection (sunscreen).7,38. Typically, the UV domain is divided into UVB ranging from 290 to 320 nm, UVA-2 between 320 and 340 nm, and UVA-1 between 340 and 400 nm. The large absorbance of TiO2 in UV is widely recognized and documented. Micron sized TiO2 particles are efficient in blocking UVB type of radiation while micron sized ZnO nanoparticles are preferentially absorbing in UVA-1 domain.28 Nevertheless, as the UV radiation is absorbed, electron-hole pairs are generated and ROS production is ignited in both ZnO and TiO2 particles. The decrease of the size of TiO2 and ZnO particles into the nanometer range increases the energy gap but this modification is not sufficiently large to make the production of pair electron-hole under the effect of the daily light negligible. Detailed investigations on the antibacterial features of ZnO/SnO2 composite films revealed that the antibacterial activity (for Escherichia coli) is enhanced by UV irradiation but it is not completely stopped in dark.18 The fact that some films retained even in the dark some antibacterial activity may indicate the presence of trapped oxygen species (assuming that a perfect dark was achieved) and may provide a path to intrinsic, passive antibacterial features (not mediated by light). A study on antibacterial features of some metal oxides (ZnO, CuO, and Fe2O3) nanoparticle revealed some antibacterial activity in all of the studied oxides, with ZnO showing the strongest antibacterial activity and Fe2O3 exhibiting the weakest antibacterial features. It was concluded that these oxides are more efficient against Gramm positive than Gramm negative strains, and that the surface appears to enhance the antibacterial features.34 No detail about the presence of light during this experiment was provided. Antibacterial features have also been reported in several semiconductors (not necessarily oxide semiconductors) such as CdS.39
Metallic and semiconducting particles and nanoparticles may exhibit antibacterial features, especially if positively charged. The electromagnetic radiation may trigger the generation of ROS under the presence of light.40 This explains the reason for which some studies confirmed the antibacterial features of these particles/nanoparticles while other researchers failed to confirm these results. Sonication may eventually replace the electromagnetic radiation in the generation of ROS in oxide semiconductors (or metals exposed to oxygen atmosphere). Aiming towards medical oriented results, it is important to conduct the experiments either under light illumination (for tissues and organs that can be easily illuminated such as the skin) or under total dark (for some internal organs). The direct production of ROS by particles or nanoparticles is not impossible. Such a situation will describe a ROS intrinsic system. However, in most cases light is required. In this case, the particle/nanoparticle plays an important role in the ROS production but is not efficient to ignite ROS generation.
ROS is a double sword. It is a collection of chemically active species (free radicals, ions, and ion-free radicals) capable of attacking and destroying biological cells at the molecular scale. From this angle, ROS is an efficient protection mechanism. However, ROS is also able to attack healthy cells, becoming dangerous to them. This process should be handled with care and activated only under certain circumstances. Therefore, a reliable procedure to turn on and off ROS can become instrumental in the protection of cells.
The cocktail of TiO2 nanoparticles and UV irradiation was used to generate ROS species in various cells, in a tentative to kill cancer cells. Preliminary tests in the laboratory demonstrated that the procedure is efficient and several improvements have already been suggested.11 Nevertheless, such an approach can be used solely for few cancers, where the activation by visible of UV light is possible. Endoscopic illumination – although possible will not convert the use of this cocktail into a treatment option for any kind of cancer but may add an important medical tool. As both Visible and UV radiation has a thin penetration depth within the human body, an important fraction of these studies are mostly theoretical.
Future research will need to disseminate between zero valence states and neutral states, to quantify accurately the effect of electromagnetic radiation and water, and in certain occasion to consider the potential effects of the oxygen presence. At least, the problem of nanoparticles (or colloids) has to be better addressed, with emphasize on surface states-where trapped positive ions can be the source of some existing discrepancies regarding antibacterial features. Specificity and efficiency remain ultimate goals before medical/human applications. ROS should be use with care as it is also considered as a major responsible for cancer (at chemical and molecular level).41
None.
None.

©2015 Chipara, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.