Journal of
eISSN: 2377-4282


Mini Review Volume 3 Issue 3
1University of California, USA
2University of British Columbia, Canada
3Baylor University, USA
4Texas A&M University, USA
Correspondence: Conrad Rizal, University of California, San Diego, La Jolla, CA, 92093, USA, Tel (858) 433-6788
Received: January 22, 2016 | Published: April 1, 2016
Citation: Rizal C, Niraula B, Lee H (2016) Bio-Magnetoplasmonics, Emerging Biomedical Technologies and Beyond. J Nanomed Res 3(3): 00059. DOI: 10.15406/jnmr.2016.03.00059
BMP, Bio-Magnetoplasmonics; MP, Magnetoplasmonics; SPs, Surface Plasmons; EM, Electromagnetic; SPR, Surface Plasmon Resonance; SPP, Surface Plasmon Polaritons; LSPs, Localized Surface Plasmons; MO, Magneto-Optical; FM, FerromagneticBMP, Bio-Magnetoplasmonics; MP, Magnetoplasmonics; SPs, Surface Plasmons; EM, Electromagnetic; SPR, Surface Plasmon Resonance; SPP, Surface Plasmon Polaritons; LSPs, Localized Surface Plasmons; MO, Magneto-Optical; FM, Ferromagnetic
Bio-magnetoplasmonics (BMP), is a relatively new field of science that employs magnetoplasmonics (MP), in biology which has a great potential application in biomedicine and biomedical technologies such as ultrafast and ultra-sensitive biosensing and bio-detection, bio-imaging, bio-therapy, drug-delivery, nano-imaging, etc.,1-4 It merges the physics of bio-nanomagnetics where biological samples such as cells and DNA are made to interact with magnetic moments, M, of a magnetic material or with applied magnetic field, H and bio-nano-optics where biological samples are made to interact with optical radiation in visible, Infrared and telecommunication wavelength ranges.5-9 In a similar manner, it merges bio-nanoplasmonics where biological samples are made to interact with surface plasmonic wave fields, also referred to as evanescent radiation fields.
The circle with big arrows (clockwise) shows a process involved in creating new MP material through innovative design, modeling, simulation and verification of these designs through nanofabrication, optical and magnetic characterization and data fitting. The outer circle (clockwise) shows the various functionalities of MPs – including biosensing, bio-imaging, Immunoassays monitoring of environmental problems, thermal therapy, space exploration, biophysics and photovoltaics. Schematic of essential characteristics of nanoparticles used in magnetoplasmonic is shown in the top right and that of multilayered nanostructure in the bottom right.
Surface plasmons (SPs) are oscillating charge density waves and are created on the surface of the materials when excited by electromagnetic (EM) radiation. Surface plasmon resonance (SPR) occurs when the wave-vector of the incident optical radiation matches that of the wave vector of the surface plasmons.10-15 Much in the same way as the SPR, in magnetoplasmonics, in the presence of magnetic materials or external magnetic fields
Depending on the device geometry and sizes, there are two types of SPs. One is the localized surface plasmons (LSPs), which are non-propagating excitation of the plasmons coupled to the EM fields and confined within the nanostructures such as metallic nanoparticles, nanorods, nanodisks, nanowires, etc., whose dimensions are smaller than the wavelength of the incident optical radiation.5,11,15-19 The other type is the surface plasmon polaritons (SPP), which arises from the coupling of the electrons at the interface between the materials with negative and positive permittivity (which is the case for metal and dielectric layers) and incident EM waves produces oscillations of electron plasma on the metallic surface that in turn generates confined two-dimensional transverse EM waves at the interface.2,20-25 These waves decay exponentially in the direction perpendicular to the interface and practically vanish at both sides of the interface. Unlike the LSPs, the SPP arises from the layered nano structured configurations where the lateral size of the nanostructure is greater than the wavelength of the optical radiation such as, multilayers of dielectric/ferromagnetic, dielectric/oxides and dielectric/plasmonic metals.
Commonly available and most popular materials used in plasmonic studies for SPR phenomena are non-magnetic (NM) noble metals such as, Au, Cu and Ag. However, being purely NM, the optical properties are determined in an extended spectral range by the conduction electron and as determined by the Drude’s theory of conduction of electron in metals, they exhibit small magneto-optical (MO) effects. The 3-d transition ferromagnetic (FM) metals such as Co, Fe and Ni and ferri-magnetic metals and their alloys and multilayers are excellent candidates for the study of MO surface plasmon resonance (MO-SPR); primarily because they offer excellent MO and optical properties such as enhanced permittivity and electromagnetic coupling and these can be controlled using both optical radiation and H fields. It is a well established fact that FM of Fe, Co and Ni offer large MO effects (e.g., MO effects in Co is ~1000 times larger than in NM metals such as Au). The propagation length of SPP calculated from Maxwell’s equation for NM metals extends in the ranges of 100-s of micrometers compared to just below 10 micrometers for the FM metals such as Co. It implies that FM metals alone cannot be used as the only metallic components of the MP system, but they can be embedded with the NM metals or oxides in the form of Core-shell or multilayered structure to increase MO effects and propagation length of the SPP. A summary of design of new MP structure and the various processes involved with these as well as about the potential applications of MPs is schematically demonstrated in Figure 1.
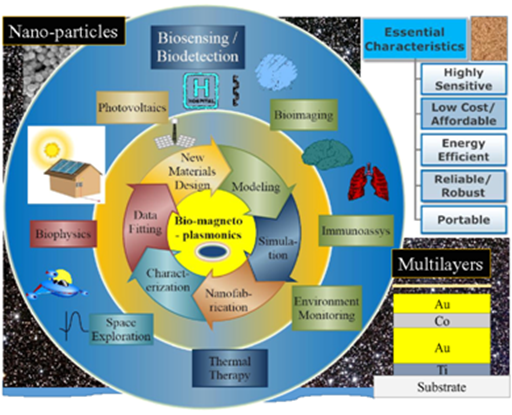
Figure 1 Schematic representation of various processes that are currently being studied within the field of magneto-plasmonics (MP) (inner circle).
SPR-based sensing using NM metals have already found commercial applications. However, detecting low concentration of biomolecules using SPR sensors is still a challenge as ultra-sensitive sensors are required for the early detection and treatment of diseases such as AIDS, tropical fevers, cancers, etc. and the signal to noise ratio of SPR sensors is limited.21,26,27 If modified by suitable magnetic materials, it is one of the most important areas where MO-SPR sensors would shine as ultra-fast, miniature and ultra-sensitive bio-sensor for early detection of chronic diseases. Since magnetic switching in FM multilayer can be achieved through optical radiation.28 and this interplay between the optical radiation and magnetic moments in the FM multilayer enables one to modify MO properties leading to high sensitivity, especially in reference to changes in refractive indices and permeability of the biological samples and the metal layers involved. For a detail review on these magnetic multilayered-based magnetoplasmonic systems, interested readers are suggested to read review articles and exciting work on MP carried out by us.29-31 as well as by Armelles and his research groups.2
The study of layered metal/dielectric-based plasmonics is a relatively new science and promises with many potential new applications in biomedicine. We recently explored new ways to enhance MO and optical modulation respectively using new ferromagnetic and dielectric layers.29,32 and theoretically demonstrated that only under an optimum design conditions, an incident optical radiation can couple to selected surface plasmon polaritons modes through the synergetic action between optical radiation and magneto-optically induced fields. This required using H fields to change the direction of magnetic moment in the sample and optical radiation at various incident angle, as external stimuli.28 It also involved miniaturization and optimization of the device structure. Combination of all these efforts lead to an increase in sensitivity of the biosensor to two order of magnitude as opposed to those conventional SPR based sensors.
Figure 2 (i) shows Au/dielectric-plasmonic nanostructures we have designed, optimized and fabricated. The SPR shift achievable by our structure is shown in Figure 2 (ii), where the minimum reflection peak shifts towards a higher θ for a protein sample. The angular shift, corresponding to the changes in ε, defines the sensitivity of the SPR configuration. Our modeling shows that the sensitivity of the SPR is not sufficient to detect a sample that has a very low protein concentration. Figure 2 (iii) shows the schematic of the new MOSPR structure that we recently designed, optimized and fabricated and Figure2 (iv) is the MOSPR effect shown by the multilayer. Instead of investigating the linear angular shifts in the SPR peaks, here we record the changes in reflectivity intensity corresponding to the changes in the permittivity of the sample when an H field is introduced. The sharp changes in the MOSPR curves result in the giant magneto-reflection (GMR) effect, defined as: [Rp (H = + H) - Rp (H = - H)]/Rp (H = 0), where Rp (H = + H), Rp (H = - H) and Rp (H = 0) denote reflections at positive, negative and zero applied H fields. Due to the tunability of light and magnetic fields, this sensor configuration can detect as small as 100 parts per billion changes in material optical properties that is 2 orders more magnitude changes in sensitivity of conventional SPR configuration. That means that, if suitably designed, fabricated and optimized, the MOSPR configuration is highly suitable for detecting samples that have extremely low protein concentration. The increased sensitivity of the MOSPR sensor is attributed to the amplified MO signal, induced by the excitation of the SPPs at the metal and dielectric interface, in response to the applied transverse H fields (the direction, as shown in the inset of Figure 2 (iv)) and transverse-magnetic polarized optical radiation. Also, Figure 2 (v-vii) show the light enhancement in non-magnetic Ag/Si multilayers that can be extended to ferromagnetic MP nanostructures for potential biomedical applications.

Figure 2 SPR and MOSPR effects:
i. Conventional SPR structure,
ii. Reflectivity vs incident angle,
iii. MOSPR structure,
iv. Sensitivity Vs incident optical angle of radiation. The inset in
v. shows the stimuli setup – It consists of basic MOSPR configuration for biosensing: A indexing matching fluid is placed in between the triangular prism and Ti buffer layer, containing 45 nm Au/Co multilayer mounted on a prism. Incident optical radiation is fed through the prism and buffer layer, and is reflected off the multilayer that passes back through the prism to a photodetector where it is collected. The changes in the reflectivity intensity versus incident angle of radiation is directly linked to the refractive index of the bio-sample attached to the multilayer surface.
vi. Optical emission enhancement in Ag/Si multilayers.
vii. .Sensitivity comparison between the SPR and MOSPR configurations.31,32
We find that these new nano-structures show distinctly new MP characteristics due to the difference in band diagrams, conductivity profile, carrier concentration and carrier mobility, Ohmic loss and magnetic orientation, to name a few. For a theory on MO layered structures, readers are suggested to read the paper by Schburt et al.33
In this mini review, the aim is to provide an overview of many recent pioneering developments in the field of nanoparticles and layered structure based surface plasmonics and magnetoplasmonics. The application of metal based SPR sensors and arrays of nano holes are showing ever growing importance of this field, see, for example, the recent works by Ertorer.34 and Menezes & Brolo.11,35 The materials and devices are equally important to study the interaction of bio-molecules with magnetoplasmonics and it is so when suitably fabricated. For example, by modifying SPR structure with the magnetic material and by varying the device structure and miniaturization, a new generation of MP material and capabilities can be created, extending the use of plasmon materials in new areas such as - optical radiation-receptor interaction, DNA hybridization, antibody characterization, epitopes mapping, label-free immuno-assay and biomarkers for diseases identification.
Photovoltaics is another area where both the light emission enhancement and extinction features have been already demonstrated using non-magnetic multilayers and metamaterial in grating configurations and this can be extended to FM multi layers based periodic arrays of nano holes.34,35
MPs create new opportunities to explore new ways to tune Surface Enhanced Raman Scattering (SERS) using magnetic core-shell nanostructures for the following: Single molecule detection, catalyzed analytical assay, in-situ ambient analysis, protein and nucleic acid, fluorescence properties, imaging, analytical genomic and proteomic studies - drug screening, immunological. A pioneering work on SERS is discussed by Brolo and his research groups.36
The application area of new MP structures is enormous. For example, new nano-magnetic structures in various geometries and configurations can be applied to MP nanoscopy (near-field scanning optical microscopy, NSOM), biomedical tests, national security, environmental monitoring, thermal phototherapy of tumours to kill cancer cells (currently on clinical trials), hydrogen sensing (using magnetic core-shell nanoparticles, for example).The use of nano-magnetic materials and ultra-fast magnetic control allows us to maximise the sensitivity, increase detection level and optical energy trapping and heating, to name a few.
Maksymov.3 and Vavasari et al.4 have recently reviewed work on MO phenomena in MP antenna such as nano-disks, nanorods, etc., that emit, receive and, control optical radiation at the sub-wavelength scale and their sizes are much smaller than the wavelength of the incident optical radiation. The fabrication method employed to grow these nanoantena is compatible with the already established complementary metal-oxide semiconductor (CMOS) manufacturing process, meaning that these can be fabricated with the industry standard CMOS fabrication process with ease. The beauty of these MP nano-antennas are that they have significant potential to be used as novel plasmon rulers and biosensors. Interested readers in review of the origin of the plasmon-enhanced MO effect are referred to research paper by Prashant & Cohen et al.18
Another MP-localised SPRs of significant interest are MO crystals - artificial structures composed of dielectric and magnetic materials with different refractive indices–that significantly affect the propagation of optical radiation. Innoe et al.1 have provided detailed theoretical reviews as well as some experimental works on MO nanocrystals. The magnetic element provides additional degree of freedom to control diffraction patterns, direction and polarization states of optical radiation, in addition to a large enhancement of Faraday rotation in materials consisted of garnet films and dielectric materials. The review also elucidates Bragg grating magnetic 2D and 3D photonic crystal based theory.
Maccaferri et al.37 have discussed details about recent work on systems allowing label-free molecular detection using MPs that have found some applications in biosensing and are expected to have enormous impact on BMP and biomedicine and deserve intense investigation in this area. We expect that especially, MP-based materials using layered structures will open further many new unforeseen phenomena and functionalities and applications to a variety of emerging technologies, especially in biomedicine and related biomedical fields. Other new devices with improved performance that could come out of MPs are light polarization rotators and non-reciprocal optical isolators that are essential building blocks in bio-nano-photonics technology. A review by Kavanagh & Gordon.38 Chin.39 and Tomnov.40 highlights details on the control of the non-reciprocal light propagation displayed by metallic and magnetoplasmonic nano-antennas and they could offer a promising route to bring these devices to the nanoscale.
The MP Layered-based devices have already generated significant interest and have demonstrated phenomena such as the giant magnetoreflectence (GMRE) effect, (similar to the giant magnetoresistance (GMR) effect shown by alloys and multi layers .30 it is a large change in reflectance, both in amplitude and phase, due to applied magnetic fields) as confirmed by our theoretical modelling and experimental work, shown in Figure 2 (for recent GMR effect and high-saturation magnetization based biosensors, interested readers are referred to papers elsewhere.41-52). The studies of MP devices and structures using FM, NM and the combination of these two, as well as using insulators and their hybrids with FM material in the form of nano structured multilayers of different configurations and geometry are believed to prompt the efforts in developing magneto-optic surface plasmon (MOSPR) amplification by stimulated emission of radiation and construction of meta-material structure at the nanoscale. Recently, Al, highly doped oxides and graphene are also being used as plasmonic materials as alternatives to Ag and Au and these can be incorporated with FM metals to create new MP materials.53-55 Since both the light emission enhancement and extinction features have been already demonstrated by non-magnetic multilayers and meta-material in grating configurations, it would be interesting to see how these effects would change when conducting oxides, e.g. ITO and IZO, are introduced into the new plasmonic nanostructure.14 the MPs possess ultra-fast switching behaviour due to controllable spin as well as being extra sensitive to applied H fields.
In summary, we introduced recently reviewed series of papers on surface and magneto plasmon structures, including our own. We believe that the review provided a fair and compressive revision of accomplishments in these research fields and about the scope and potential application of magnetoplasmonics. It is a well acknowledged fact that the future of magneto-plasmonic based nanostructures is extremely bright. The exceptional properties displayed by magnetoplasmonic based nanostructures, such as strong enhancement of electromagnetic fields, high sensitivity, large signal to noise ratio, possibility of obtaining high photo-thermal conversion efficiencies and rich spectral responses at applied H fields, make them unique, outstanding and sought for material for various applications. Their potential application may range from diagnostics, clinical therapy, bio-imaging, biophysics, environmental monitoring, ultra-sensitive and ultra-fast molecular sensing for early disease detection, chemical and biological sensing, magneto-plasmon-enabled photo-thermal therapy, magneto-plasmon-assisted laser welding, plasmon-assisted photo-acoustic imaging and magneto-plasmon-enhanced spectroscopies, such as the SERS for magneto-plasmonic structures and the field is expected to extend to the energy production and as well as in space exploration.
Additional areas where MP-based devices and structures shine are magneto-plasmon enhanced photo-detectors, isolators, solar energy harvesting and conversion and coupling of magneto-plasmons to chemical reactions for achieving high activity and selectivity for energy-saving, femto second switching and sensing and tuning of magneto-optical properties at the femtosecond speed. These areas are highly promising to reach their full potential in new magnetoplasmonic-based technologies - including development of bio-nanomagnetic and magnetoplasmonic bioengineering, high-performance magnetronic devices and green energy, and biology and biomedicine, among many others.
None.
None.

©2016 Rizal, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.