Journal of
eISSN: 2373-6453


Research Article Volume 2 Issue 4
1Department of Biochemistry, University of Wurzburg, Germany
2Genelux Corporation, San Diego Science Center, USA
3Department of Radiation Medicine and Applied Sciences, University of California, USA
4Rudolf Virchow Center for Experimental Biomedicine, University of Würzburg, Germany
5Institute for Molecular Infection Biology, University of Wurzburg, Germany
Correspondence: Aladar A Szalay, Department of Biochemistry, Rudolph Virchow Center for Experimental Biomedicine, Institute for Molecular Infection Biology, University of Wurzburg, Germany, Tel 1.858.200.6586
Received: May 07, 2015 | Published: May 18, 2015
Citation: Ehrig K, Chen NG, Stritzker J, Kilinc MO, Buckel L, et al. (2015) Vaccinia Virus-Mediated Expression of Transcription Factor Klf4 Enhances Oncolytic Virotherapy of Colorectal Cancer. J Hum Virol Retrovirol 2(4): 00051. DOI: 10.15406/jhvrv.2015.02.00051
Colorectal cancer (CRC) detected at an early stage has a good prognosis, however, recurrence of the disease is prevalent and late stage CRC (stage IIIC and IV)has observed 5-year survival rates of 28% and 6%, respectively. Oncolytic virotherapy has been explored as a potential treatment modalityin cancer therapy for its safety and tumor specificity. We recently reported that oncolytic vaccinia virusGLV-1h68 efficiently infected, replicated in, and lysed different colorectal cancer cell lines derived from patients at different stages of the disease in culture and mouse xenograft models. However, the cytotoxicity of GLV-1h68 was cell line-dependent and, in particular, xenograft tumors of the colorectal cancer cell line HT-29 did not respond favorably to oncolytic treatment with GLV-1h68. Here we report on the generation of recombinant vaccinia viruses expressing the colorectal tumor suppressor Klf4. We show that a single injection of vaccinia virus expressing Klf4 led to significant tumor growth inhibitionofHT-29 human colon cancer xenografts in comparison to treatment with GLV-1h68. Furthermore, virus-mediated expression of a membrane-permeable Klf4-TAT fusion protein, further improved the anti-tumoral effects. This is the first report demonstrating that arming oncolytic viruses with the colorectal tumor suppressor Klf4 led to improved therapy in a human colon cancer xenograft model.
Keywords:Vaccinia virus, Oncolytic virotherapy, Klf4, Colorectal cancer, Transcription factor
Klfs, Krueppel-Like Factors; Klf4, Krueppel-Like Factor 4; Hh, Hedge Hog; FAP, Familial Adenomatous Polyposis; ORFs, Open Reading Frames; BMP, Bone Morphogenetic Protein
The standard treatment for in situ or localized colorectal cancer is surgical excision of the primary tumor, while treatment of TNM stage III colorectal cancer usually also requires adjuvant chemotherapy. Combination therapy increases 5-year survival rates of stage III patients to 73%. However, recurrence rates remain around 40-60% in the first three years after surgical excision and chemotherapy, likely caused by chemo- and radiation-resistant cancer-initiating cells. Complicating the treatment of colorectal cancer is the variation and complexity inherent in the etiology of colorectal cancers. Neoplastic transformation of CRC is thought to be due to the accumulation of inherited or acquired DNA mutations, most commonly in the Wnt (through mutations in the apcor β-catenin genes), TGF-β, Notch and hedgehog (Hh) signaling pathways.1,2
The complexity of these signaling networks and the various genetic alterations that influence the cellular balance to keep normal epithelial cells in check make successful treatment of the disease challenging. The need for novel efficient and safe treatments for patients diagnosed with recurring or late stage CRC disease is of great need. Krueppel-like factor 4 (Klf4) is a member of the Krueppel-like family of zinc finger transcription factors3. Krueppel-like factors (Klfs) regulate proliferation, differentiation, development and apoptosis. Klf4 acts as both a transcriptional activator (klf4, p21WAF1/Cip1, alkaline phosphatase) or a repressor (cyp1a1, cyclin D1/B1/E)and is most abundant in epithelial tissues of the intestinal tract and the skin, and in vascular endothelial cells and the thymus.3-5 Klf4 regulates cell growth of intestinal cells and terminal differentiation of selected epithelial tissues.6 It is highly expressed in terminally differentiated, quiescent post-mitotic upper crypt epithelial cells of the colonic mucosa. Klf4 expression in culture is increased under growth arrest promoting conditions (i.e. DNA damage, serum deprivation, contact inhibition) and leads to inhibition of DNA synthesis and cell cycle arrest7 and down-regulation of Klf4 may be associated with uninhibited cell growth and malignant transformation.
The activities of Klf4 suggest that it may have tumor suppressor function. Levels of klf4 mRNA transcript are significantly reduced in patients with familial adenomatous polyposis (FAP) and other colorectal cancers compared to normal intestinal tissues.8 Loss of heterozygosity of the klf4 locus, mutations in open reading frames (ORFs), and hyper methylation of klf4 promoters have been identified in various human CRCs and cell lines.9 Klf4 also inhibits the transcription of nuclear β-catenin by binding to its C-terminal trans activating domain, implicating the role of Klf4 in the maintenance of intestinal homeostasis and the inhibition of colorectal carcinogenesis.10 In fact, colorectal cancer lines with low levels of endogenous Klf4 expression exhibited diminished tumorigenicity when induced to express Klf4.11 Gradual increase in Klf4 expression correlates with loss of hyper proliferation and greater phenotypical differentiation of HT-29 cells in response to short-chain fatty acid stimulation.12 Klf4 expression also correlates significantly with the degree of differentiation in colorectal cancer specimens from CRC patients.13 Thus, Klf4 may be a useful tool to promote the reduction of tumor cell growth in colorectal cancer.
The applicability of oncolytic virotherapy has been studied preclinically and in clinical trials with different viruses. The inherent ability to rapidly replicate in, and lyse human tumor cells in comparison with other viruses as well as its large foreign gene-carrying capacity make vaccinia virus a leading candidate for use in cancer therapy.14 We previously reported on the tumor selectivity and anti-tumoral efficacy of the replication-competent vaccinia virus strain GLV-1h68 in a broad variety of human tumor xenograft models.15 More recently, we demonstrated the functional applicability of “second-generation” recombinant vaccinia virus (rVACV) strains expressing therapeutical payloads such as anti-VEGF single-chain antibodies targeting tumor angiogenesis and radio sensitization of the tumor-associated vasculature,16,17 the human sodium iodide symporter (hNIS),18 enzymes for the synthesis of melanin for optoacoustic deep tissue imaging and laser-induced thermotherapy of cancer19 or bone morphogenetic protein-4 (BMP4) in an orthotopic glioblastoma model.20
In this study, their VACV strainsGLV-1h291 and GLV-1h391 containing klf4 cDNAs were constructed and their potential effects on oncolytic virotherapy of colorectal cancer were evaluated. We demonstrated the functional expression of Klf4 and membrane-permeable Klf4-TAT fusion proteins by infected HT-29 human colorectal cancer cells. In contrast to our previous results that GLV-1h68 failed to inhibit tumor growth of HT-29 human colorectal cancer xenograft tumors in mice,21 here we report that systemic delivery of klf4-expressingr VACV strains GLV-1h291 and GLV-1h391 into mice with established HT-29 non-responder colorectal xenograft tumors led to significant tumor growth inhibition. Strongest tumor growth inhibition and longest overall survival were observed in animals treated with GLV-1h391 encoding the membrane-permeable klf4-TAT-fusion product. We propose that rVACV-mediated expression of membrane-permeable Klf4-TAT decreases intracellular β-catenin levels and promotes local cell growth arrest in neighboring cells, thus slowing down tumor progression.
Klf4 expression in HT-29 cells infected with Klf4-encoding rVACV strains
After generation of the klf4-expressingrVACV strains GLV-1h291 and GLV-1h391 (Figure 1a), the expression of Klf4 in virus-infected HT-29 cells was analyzed by Western blot immuno detection using anti-Klf4 polyclonal antibodies. HT-29 cells were either mock-infected, infected with GLV-1h68 as a control, or one of the klf4-expressing viruses, GLV-1h291 (Klf4) and GLV-1h391 (Klf4-TAT). Klf4 was clearly detectable 24 hours post infection in GLV-1h291- or GLV-1h391-infected HT-29 cells in culture (Figure 1b). Mock-infected and GLV-1h68-infected cells showed little to no Klf4 expression. As expected, the Klf4-TAT fusion protein was slightly greater in relative molecular mass than the native untagged Klf4.
Klf4 expression from Klf4-encoding rVACV strains GLV-1h291 and GLV-1h391 was also analyzed by immunofluorescence 24 hours post-infection (Figure 1c). HT-29 cells were infected with klf4-expressing GLV-1h291 or GLV-1h391, or GLV-1h68 for 24 hours. Cell nuclei were then stained with Hoechst and the cellular location of Klf4 and Klf4-TAT was determined by immunofluorescence microscopy. Isolated plaques were identified by GFP detection. Klf4 expression was detected in cells infected with GLV-1h291 and GLV-1h391. No Klf4 was detected in mock-infected or GLV-1h68-infected cells. Merged Klf4 and Hoechst staining signals demonstrated nuclear localization of the Klf4 protein, indicating that virus-encoded klf4 was efficiently translocated to the nucleus, and that the fusion of the C-terminal TAT sequence to Klf4 did not affect its translocation.

Figure 1 Detection of Klf4 in virus infected cells.
Functionality of virally expressed Klf4
Klf4 inhibits β-catenin-mediated transcription by binding to the C-terminal trans activating domain of the β-cateninprotein10. HT-29 cells infected with klf4-expressingrVACVs at a high MOI (5) were analyzed by qPCR (Figure 2a & Table 1) and ELISA (Figure 2b) to determine the transcription and expression levels of β-catenin after infection. A significant increase in klf4 mRNA transcript levels (P≤0.001) in HT-29 cells infected with klf4-expressing rVACV strains at 24 hpi (Table 1) coincided with a complete reduction of gusA mRNA levels, indicating that the glucoronidase gene cassette was successfully replaced by the klf4 or klf4-TAT gene cassette (Figure 1a). Notably, a significant decrease (P≤0.05) in β-catenin mRNA levels in cells infected with either GLV-1h 291 (3.0 ± 2.0%) or GLV-1h391 (21.9 ± 2.3%) was observed when compared to HT-29 cells infected with the GLV-1h68 virus strain. Similarly at 48 hpi, cellular β-catenin protein levels were significantly (P≤0.005) decreased to 74.0 ± 5.2% and 76.3 ± 2.7% in klf4-expressing GLV-1h291- and GLV-1h391-infected cells, respectively, compared to GLV-1h68 (Fig. 2b).
Gene |
GLV-1h68 |
GLV-1h291 |
GLV-1h391 |
||||
mean ± s.d. |
P |
mean ± s.d. |
P |
mean ± s.d. |
P |
||
VACV |
A21L |
100.0 ± 24.9 |
18.0± 2.6 |
* |
45.8 ± 2.5 |
* |
|
lacZ |
100.0 ± 27.5 |
17.9 ± 3.5 |
* |
24.6 ± 5.3 |
* |
||
gusA |
100.0 ± 15.0 |
0.0 ± 0.0 |
** |
0.0 ± 0.0 |
** |
||
klf4 |
100 ± 21.4 |
5.1x104± 4.1x103 |
*** |
9.4x104 ± 2.5x104 |
** |
||
host cell |
β-catenin |
100.0 ± 37.3 |
3.0± 2.0 |
** |
21.9 ± 2.3 |
** |
|
cyclin D1 |
100.0 ± 17.7 |
28.6± 11.6 |
* |
55.5 ± 10.7 |
* |
||
Gapdh |
100.0 ± 25.6 |
45.3 ± 18.0 |
* |
141.0 ± 30.5 |
|||
Table 1 Confluent HT-29 cells were infected for 24 hours at an MOI of 5.0 with GLV-1h68 or klf4-expressing GLV-1h291 or GLV-1h391
Confluent HT-29 cells were infected for 24 hours at an MOI of 5.0 with GLV-1h68 or klf4-expressing GLV-1h291 or GLV-1h391. Results are presented as relative percentages of expression in comparison to expression measured in cells infected with GLV-1h68 to account for the effects of rVACV infection alone. The mRNA transcript level of vaccinia virus-related genes (a21l, lacZ, gusA and klf4) and host cell genes (β-catenin and cyclin D1 for Klf4 functionality, gapdh as housekeeping gene) were analyzed by the comparative CT (2-ΔΔCT) method and corrected against 18SRNA. * = P≤0.05, ** = P≤0.001, *** = P≤0.0001
Further analysis was performed by qPCR to examine the effect of virally expressed Klf4 on the repression of cyclin D1 expression. To avoid false positive/negative data from a mixed population of infected/uninfected cells, HT-29 cells were synchronously infected at a high MOI (5.0) to ensure infection of all cells at the same time. After 24 hours of synchronized infection, cyclin D1 mRNA transcript levels were 28.6 ± 11.6% (P≤0.05) and 55.5 ± 10.7% in GLV-1h291- and GLV-1h391-infected cells, respectively, compared GLV-1h68-infected cells (Table 1). The relatively low level of gapdh (housekeeping gene) transcript in GLV-1h291-infected cells (45.3 ± 18.0%) and the relatively high level in GLV-1h391-infected cells (141.0 ± 30.5%) as compared to GLV-1h68-infected cells correlated with the observed differences in cytotoxicity of these virus strains in HT-29 cells in culture (Figure 3a).
Notably, significant differences in β-catenin and cyclin D1 mRNA levels in Klf4-expressing virus-infected cells were observed despite the reduced levels of transcription of the viral gene A21L as well as virally-expressed lacZ, indicating lower viral replication compared to the control strain GLV-1h68 (Table 1). The lower levels of transcription of virally-expressed genes in klf4-expressingr VACV strains correlated with inferior levels of replication (Figure 3b). Our findings suggest that virus-expressed Klf4 in infected cells is functional and, importantly, that the C-terminal TAT fusion does not interfere with the binding of Klf4 to the C-terminally located trans activation domain of β-catenin10.
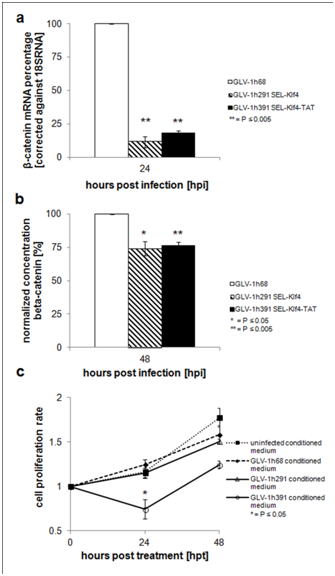
Figure 2 Functionality of Klf4 or Klf4-TAT protein expressed in virus-infected cells.

Figure 3 Cytotoxicity, replication and induction of apoptosis of GLV-1h291 (Klf4) and GLV1h391 (Klf4-TAT) virus strains in HT-29 cells.
Functionality of C-terminally fused TAT transduction domain in Klf4
To assess whether the Klf4-TAT fusion protein is able to translocate through cytoplasmic membranes and cause the arrest of cell proliferation, HT-29 cells were cultured at a low density with conditioned media containing either Klf4 or Klf4-TAT from virus-infected CV-1 cell lysates. After culturing the cells for 24 h significant inhibition of cell proliferation (P≤0.05) was evident in cells cultured with the conditioned medium containing Klf4-TAT (Figure 2c) while cells cultured with conditioned media containingklf4-expressing
GLV-1h291-infected cell lysates showed no reduction in proliferation. Conditioned media containing mock-infected or GLV-1h68-infected cell lysates also had no effect on cell proliferation. These findings strongly indicate the functionality of the TAT transduction domain fused to the Klf4 in enabling the transcription factor to enter cells by passage through the cytoplasmic membrane and initiate cell growth arrest.
In vitro replication and cytotoxicity of klf4-expressing GLV-1h291 and GLV-1h391 in HT-29 cells
Thus, the two klf4-expressingrVACV strains GLV-1h291 (Klf4) and GLV-1h391 (Klf4-TAT) exhibited strong Klf4 expression and down-regulation of cellular β-catenin levels in infected HT-29 cells. In addition, GLV-1h391 infected cell lysates proved potent in inducing cell growth arrest of HT-29 cells in culture. Therefore, both virus strains were next analyzed for their replication ability and cytotoxic potential in comparison to the control strain GLV-1h68. The results indicated that both virus strains GLV-1h291 (Klf4) and GLV-1h391 (Klf4-TAT) replicated similarly in HT-29 cells. After virus infection at a MOI of 0.01 (Figure 3b), a three-log increase in virus titers was observed from 6 to48hpi, and remained at a consistent high level there after. However, this level of replication was inferior to that of GLV-1h68. The relative replication efficiencies of the klf4-expressing viruses were further analyzed by infecting HT-29 cells at a high MOI (5.0) to synchronize the infection. After 24 hours of infection, again the virus titers of the control strain GLV-1h68 in HT-29 cells infected at a high MOI were statistically significantly higher (P≤0.05) than both klf4-expressing virus strains, GLV-1h291 andGLV-1h391.
Cell viability assays of virus-infected HT-29 cells were performed to determine whether viral infection leads to cell lysis (Figure 3a). Interestingly, we found that the klf4-TAT-encoding rVACV strain GLV-1h391 was significantly (P≤0.0005) less cytotoxic compared to GLV-1h68 and GLV-1h291. As compared to infection at an MOI of 1.0 with GLV-1h68 and GLV-1h291, both of which efficiently lysed HT-29 cells in culture, cells infected with GLV-1h391 showed a decrease in cytotoxicity over the course of 72 hours of infection. The difference was obvious after only 24 hours of infection (109.4% ± 4.7% viable in GLV-1h391-infected cells at an MOI of 1.0 compared to 63.6 ± 0.7% in GLV-1h68-infected cells and51.3 ± 0.2% in GLV-1h291-infected cells). After 48 hours, the cytotoxic effect of infection with GLV-1h68 (10.0 ± 1.8% viable) and GLV-1h291 (8.2 ± 1.7% viable) was significantly greater than infection with GLV-1h391 (60.6 ± 1.9% viable).
The time required for 50% lethality at a dose of MOI 1.0 was extrapolated to 26.5 and 24 hours for GLV-1h68 and GLV-1h291, respectively. However, the time required to kill 50% of the cells after infection with GLV-1h391 at MOI 1.0 doubled to 51.5 hours. After 48 hours of infection at a low MOI (0.01), infection with GLV-1h291 was significantly more cytotoxic to HT-29 cells than GLV-1h68 (75.7 ± 1.4% viable in GLV-1h291-infected cells compared to 90.3 ± 2.4% viable in GLV-1h68-infected cells) (Figure S1). Cytotoxicity did not significantly differ between GLV-1h68 and GLV-1h291 when HT-29 cells were infected at a high MOI (5.0, synchronized infection). In summary, the Klf4-TAT expressing virus strain GLV-1h391 exerted a cytotoxic effect on HT-29 cells upon infection, but was less cytotoxic thanGLV-1h68 or GLV-1h291. Additionally, while comparable at higher MOIs, GLV-1h291 killed HT-29 cells in culture more efficiently than the GLV-1h68 strain at a low MOI (0.01).
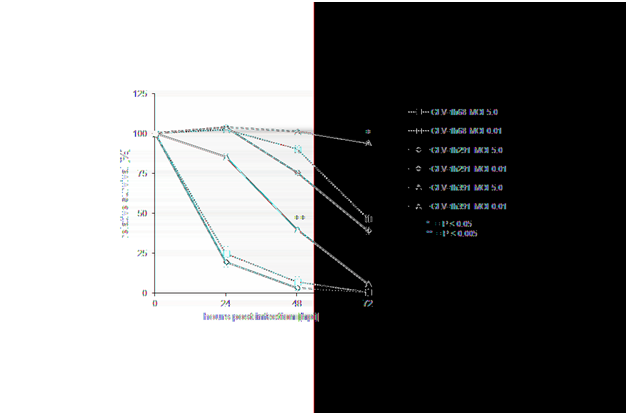
Supplementary Figure 1 Detailed analysis of VACV cytotoxicity in HT-29 cells.
For a detailed analysis of the difference in cytotoxicity of the klf4-expressing vaccinia virus strains, confluent HT-29 cells were infected at an MOI of 0.01 or 5.0. Again, cell viability was monitored over the course of 72 hours, measured in triplicate. Values are normalized against uninfected controls at each time point which were considered 100% viable. * = P≤0.05, ** = P≤0.005
HT-29 cells express a mutated p53 (R273H) protein which abolishes the p53-dependent apoptotic pathway. On the contrary, R273H p53 has been shown to inhibit apoptosis through transcriptional activation of genes like NFKB2 or BCL2L1.22,23 In HT-29 cells infected for 24, 48, and 72 hours with GLV-1h68 and GLV-1h291, similar distributions of total live, early- and late-apoptotic cells were observed (Figure 3c). Over the course of 72 hours, more HT-29 cells became infected and subsequently progressed through an early apoptotic state (AnnexinV pos/7-AADneg) into the late apoptotic state (AnnexinV pos/7-AADpos). Interestingly, after 24 hours of infection, a much lower number of total cells infected with GLV-1h391 (6.83% in GLV-1h391-infected cells, 39.51% in GLV-1h291-infected cells, and 54.62% in GLV-1h68-infected cells) were apoptotic when compared to cells infected with GLV-1h68 or GLV-1h291. Our finding that HT-29 cells infected with GLV-1h391 displayed less apoptosis than GLV-1h68 or GLV-1h291 correlated well with our findings that GLV-1h391 appeared less cytotoxic.
In conclusion, the cytotoxicity in infected HT-29 cells associated with Klf4-expressing GLV-1h291 and GLV-1h68, compared to the Klf4-TAT-expressing GLV-1h391 strain, which was much less, was not simply a function of their viral replication efficiency, since both Klf4-expressing viruses replicated at levels similar to each other and at levels much less than GLV-1h68. In vivo effects of Klf4 and Klf4-TAT expressing rVACV strains on the growth of HT-29 non-responder xenograft tumors in mice. Despite the long-standing laboratory experience with vaccinia virus, there is no established method by which to predict the outcome of vaccinia virus infection on cancer cells in vivo. We previously demonstrated that rVACV strain GLV-1h68 infects replicates in and lyses different colorectal cancer cell lines in culture, including HT-29.24 Nevertheless, xenograft tumors produced with HT-29 cells displayed a non-responder behavior in vivo21. Understanding and overcoming this behavior would be extremely valuable as oncolytic virotherapy advances into human clinical use. The expression of additional bio functional payloads by recombinant vaccinia viruses could be one method to overcome specific in vivo limitations.
Whether virus-mediated expression of Klf4 (GLV-1h291) or Klf4-TAT (GLV-1h391)enhances anti-tumoral efficacy of vaccinia virus-based oncolytic virotherapy in vivo was evaluated in HT-29 tumor-bearing mice injected intravenously with a single dose of 1x106 PFU of either GLV-1h68, GLV-1h291 (Klf4) or GLV-1h391 (Klf4-TAT). Starting from 14 dpi, both GLV-1h291 and GLV-1h391significantly (P≤0.05 for GLV-1h291, P≤0.005 for GLV-1h391) inhibited tumor growth compared to untreated controls and GLV-1h68-treated animals (Figure 4 & Figure S2). Between GLV-1h291 and GLV-1h391 treatment groups, tumor growth inhibition was greater in the Klf4-TAT-expressing GLV-1h391 group, becoming statistically significant 28 days after treatment (P≤0.05). A slight decrease in net body weight was observed after virus injection but the treatment was generally well tolerated (Figure S3). Furthermore, mean group survival 49 days after treatment was higher in the GLV-1h391 treatment group (50%) compared to untreated (0%) and the other virus-treated groups (10% in GLV-1h68 and GLV-1h291-treated animals) (Figure 5a). Log Rank testing showed that only the GLV-1h391 treatment group significantly increased survival when compared to untreated controls (P≤0.005). The mean time to reach 10 times the initial starting tumor volume (FTV = 10V (0)) was calculated for each of the groups. Overall, GLV-1h291-treated animals showed a 4.0-day gain in FTV over untreated animals and a 2.5-day gain over GLV-1h68-treated animals. Animals treated with GLV-1h391 showed a 7.2-day gain over untreated and a 5.6-day gain over GLV-1h68-treated animals, making it the most efficient treatment regimen of all tested groups (Figure 5b).
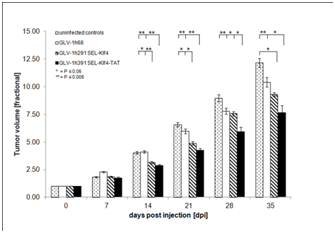
Figure 4 Effects of klf4-expressingoncolytic rVACV strains on tumor growth in HT-29 tumor-bearing mice.
HT-29 cells were implanted subcutaneously in the right hind leg of athymic nude mice and injection with Klf4 or Klf4-TAT-expressingrVACV strains was compared to mock-injection (PBS) or injection with GLV-1h68. Mean fractional tumor volume (FTV) ± SEM of [n=10 for virus-treated, n=5 for PBS treated controls] mice is plotted and one-way analysis of variance (ANOVA) was used to compare the data for each treatment group. Lower asterisks represent significance for untreated or GLV-1h68 vs. GLV-1h291 treatment groups while upper asterisks represent significance for untreated or GLV-1h68 or GLV-1h291 vs. GLV-1h391 treatment groups. P≤0.05 was considered statistically significant; * = P≤0.05, ** P≤0.005
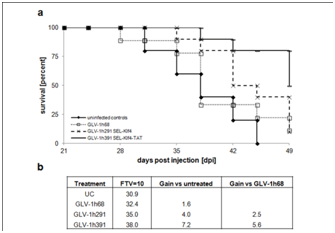
Figure 5 Survival benefits of klf4-expressingrVACV-treated HT-29 tumor-bearing mice.
Survival rates of untreated and virus-treated mice were analyzed over the course of 49 days and plotted as a survival diagram
In addition, Renilla luciferase signal of five animals, randomly chosen at the beginning of the study, was quantified at 14 and 28 dpi (Table S1). Quantification of Renilla luciferase signals showed comparable average mean intensities 14 and 28 dpi with GLV-1h291 or GLV-1h391 compared to GLV-1h68-treated animals. Also, there was no significant difference between treatment groups in virus recovered from the tumors 21 dpi (after in vivo viral replication) (Figure S4), suggesting that virus replication alone was not the cause of reduced tumor size in GLV-1h291 and GLV-1h391-treated mice as compared to GLV-1h68.
14 dpi |
|
28 dpi |
|
|
rVACV strain |
Average Mean Intensity |
# |
Average Mean Intensity |
# |
GLV-1h68 |
17.79 ± 9.86 |
3/5 |
34.82 ± 38.48 |
4/5 |
GLV-1h291 |
22.46 ± 11.65 |
5/5 |
35.22 ± 15.55 |
5/5 |
GLV-1h391 |
18.97 ± 8.65 |
5/5 |
35.21 ± 13.74 |
4/4 |
Table S1 Quantification of Renilla luciferase signals in rVACV-injected HT-29 tumor-bearing mice
Fourteen and 28 days after virus injection, animals were injected retro orbitally (r.o.) with 25 µg benzyl-coelenterazine / 100 µL PBS and imaged using a Care stream Imaging machine for 1 min. Renilla luciferase signal strength is plotted as average mean intensity with standard deviations for each virus-treated group 14 and 28 days post injection (all animals included). The # column of the table indicates the number of animals with positive signals out of the total animals tested in the group.
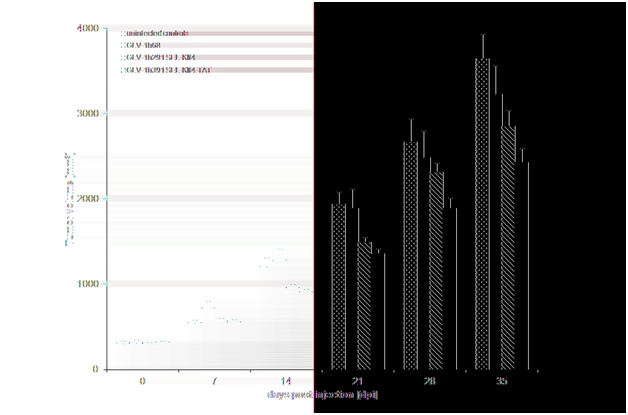
Supplementary Figure 2 Effects of klf4-expressing oncolytic rVACV strains on tumor growth in HT-29 tumor-bearing mice.
HT-29 cells were implanted subcutaneously in the right hind leg of athymic nude mice and injection with Klf4 or Klf4-TAT-expressing rVACV strains was compared to mock-injection (PBS) or injection with GLV-1h68. Absolute tumor volume (mm3) + SEM of [n=10 for virus-treated, n=5 for PBS treated controls] mice is plotted.
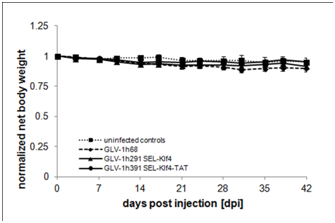
Supplementary Figure 3 Changes in net body weight after rVACV injection.
Net body weight (g) of rVACV-injected animals was calculated using the following formula, body weight (g) – (tumor volume/1000 mm3) and normalized against initial body weight at day 0 when the animals were injected with virus.
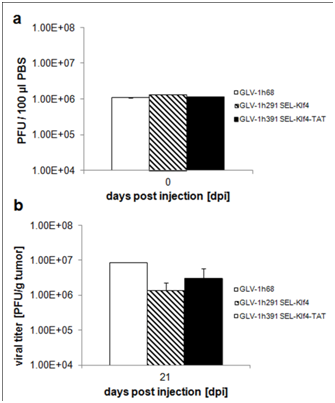
Supplementary Figure 4 Initial rVACV titers and viral distribution in HT-29 tumor-bearing animals.
The colonization of the primary tumors by the oncolytic vaccinia viruses was evaluated in more detail in animals of the different treatment groups sacrificed at 21 dpi, when the differences in tumor volumes between groups were statistically significant for seven days. Additionally, the primary tumors were surgically removed and sections were immuno histochemically stained for Klf4 and cellular actin. Klf4 expression was exclusively detected in tumors from animals treated with GLV-1h291 or GLV-1h391. Analysis of GFP expression confirmed that all three tested viruses, including GLV-1h68, efficiently colonized the subcutaneously implanted HT-29 tumors (Figure 6). Our findings, that only treatment groups expressing Klf4 or Klf4-TATled to significant tumor inhibition suggests a contributing role of Klf4 in colorectal tumor suppression.
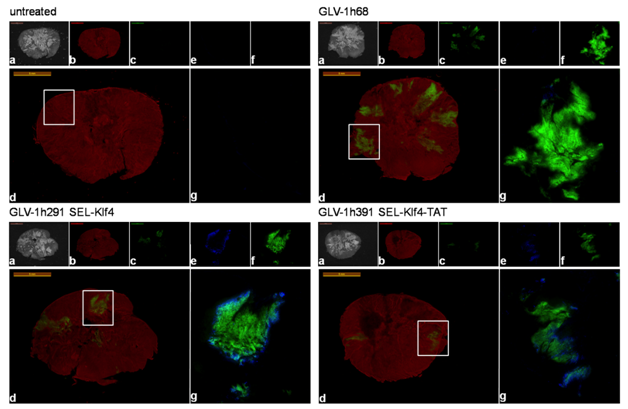
Figure 6 Immunohistochemical analysis of klf4-expressingrVACV-treated HT-29 tumor sections.
Untreated, GLV-1h68- and klf4-expressingvaccinia virus-treated mice were sacrificed 21 dpi. Whole tumor cross-sections
In the industrialized world, CRC is amongst the most abundant cancers. Despite a generally good prognosis after treatment with standard therapy regimens, diagnosis of stage IV colorectal cancer still results in poor overall outcome. Moreover, invasive procedures like surgical excision or chemotherapy treatment pose a considerable burden on patient health. Therefore, the development of novel, tolerable and efficient trategies for the treatment of colorectal cancer is still of great importance. Advancements in oncolytic virotherapy have enabled several viruses25-29 to be clinically tested for treatment of colorectal cancer. However, for many oncolytic viruses, a major drawback is the need for regional or local delivery. We recently described that systemically delivered oncolytic vaccinia virus GLV-1h68 efficiently inhibited tumor growth in subcutaneous xenograft CRCs of different clinical stages but that viral replication efficiency and cytotoxicity in CRCs was MOI- and cell line-dependent 24, suggesting that therapeutic outcomes with certain cancers might be variable. Recently, new approaches to treat CRCs have targeted regulatory or signaling pathways with tumor suppressing activities, such as the Wnt pathway through TAK1 inhibition,30 inhibition of the β-catenin/TCF-mediated transcriptional activity by resveratrol,31 inhibition of β-catenin mRNA transcription by berberine32 or stabilization of the β-catenin destruction complex by small molecules.33
Of particular interest is the transcription factor Klf4. Inducible expression of Klf4in a stably transfected colorectal RKO cell line was shown to inhibit cell proliferation by blocking cell cycle progression34 whereas down-regulation of Klf4 resulted in uninhibited cell growth and malignant transformation in various patient CRCs and cell lines.9 Klf4 targets β-catenin and inhibits Wnt signaling to maintain intestinal homeostasis10 and its re-expression leads to diminished tumorigenicity in CRClines11, making it an intriguing option for colorectal cancer-targeted arming of vaccinia virus. It has recently been reported that Klf4 can also act as a tumor suppressor in different cancer models like renal cell carcinoma,35 hepatocellular carcinoma36 or squamous cell carcinoma.37
Here, we engineered klf4-encoding rVACV strains to analyze the effects of virus-mediated (membrane-permeable) Klf4 expression in CRC cells. Our previous work showed that the rVACV strain GLV-1h68 infected, replicated in, and lysed HT-29 cells in culture and invaded and colonized subcutaneous HT-29 tumors in xenograft mouse models but failed to inhibit tumor growth. Thus, HT-29 tumors were designated as non-responders to GLV-1h68 oncolytic treatment in this model and could serve to evaluate modified GLV strains for improved therapeutic efficacy. One such modification was the virus-encoded expression of Klf4. For HT-29 tumors in which the oncolytic activity of GLV-1h68, for example, may not be optimal, high level and coordinated expression of virus-encoded Klf4 could be of major benefit.
Cell-penetrating peptides have been studied extensively as tools for intracellular delivery of therapeutic peptides.38 Among the most studied, the protein transduction domain from the human immunodeficiency virus TAT protein results in delivery of biologically active fusion proteins in vivo to tissues in mice.39 A Klf4-TAT fusion protein, then, if expressed in infected tumor cells, could enable the delivery of Klf4 to adjacent, uninfected tumor cells. To test this, we constructed recombinant vaccinia viruses by introducing into GLV-1h68 a transgene encoding either the native klf4 gene or a gene encoding Klf4 with a C-terminally fused TAT transduction domain sequence, both controlled by the VACV synthetic early/late promoter (SEL). We demonstrated efficient protein expression in HT-29 cells for both Klf4-expressing rVACV strains (GLV-1h291 and GLV-1h391). Klf4 was located in the cell nucleus, indicating correct protein folding and transportation through the pores of the nuclear membrane. It was demonstrated by Zhang et al.10 that Klf4 interacts with β-catenin protein in the nucleus and inhibits Wnt/β-catenin-mediated signaling and transcription by binding to the trans activation domain10. The HT-29 cell line contains two carboxyl terminal-truncated APC proteins instead of a functional APC protein40 leading to overly abundant intracellular β-catenin levels and increased β-catenin-mediated transcription. Furthermore, Wnt signaling triggers a LEF-1 positive feedback loop to enhance by 100-300% the nuclear chromatin-retained pool of β-catenin.41
Our studies showed that Klf4 expression was inversely related to cyclin D1 mRNA and β-catenin mRNA levels as well as β-catenin protein levels in infected HT-29 cells. These findings indicate the functionality of virally expressed Klf4 and suggest its possible role as a CRC tumor suppressor. Virus-mediated expression of Klf4-TAT by GLV-1h391 led to a comparable decrease in cellular β-catenin when compared to native Klf4, indicating that the C-terminal fusion of the TAT transduction domain does not interfere with the C-terminally located zinc finger motifs or the NLS 3. Both klf4-expressing virus strains showed Klf4-mediated effects in HT-29 cells in culture. Overall, infection of HT-29 cells with GLV-1h291 or GLV-1h391 resulted in lower β-catenin levels than GLV-1h68, even though their actual virus replication was inferior. Interestingly, while the cytotoxicity of infection of HT-29 cells was greatest with GLV-1h291, infection with GLV-1h391 led to an approximately 10-fold decrease in cytotoxicity when compared to GLV-1h68 or klf4-expressingGLV-1h291 strains. We suggest that virus infection of host cells and subsequent expression of membrane-permeable Klf4-TAT allows the recombinant protein to enter adjacent, uninfected cells and promote cell growth arrest and quiescence, both of which deter VACV infection.
While vaccinia virus’ mode of cell entry and expression of early genes are independent of the cell proliferative status, replication of thymidine kinase-negative (TKneg) vaccinia virus strains like GLV-1h68 and its derivatives is dependent on the host cell expression of TK to provide the necessary building blocks for viral DNA synthesis. Host cell translation of thymidine kinase mRNA is 10-fold higher in S phase than in G1/G0 of the cell cycle and TK mRNA is rapidly cleared after cell division, making cellular thymdine kinase expression very transient. Even though vaccinia virus growth factor (VGF) promotes the transition of cells into a proliferative state, expression of membrane-permeable Klf4 could drive uninfected cells towards growth arrest and quiescence, countering the effects of VGF. The observations that Klf4-TAT in conditioned media from virus-infected cells considerably decreased cell proliferation and both, GLV-1h291 and GLV-1h391 both showed impaired virus replication when compared to GLV-1h68 at either low or high MOIs, further supports this notion.
It is noteworthy, that Klf4 can act as a transcriptional repressor of p53 by directly acting on its promoter. Expression of Klf4 in HT-29 cells could thus potentially have an oncogenic effect by impeding p53 expression and, consequently, function. However, it has been shown that HT-29 exhibits a R273H point mutation in the p53 gene, abrogating the tumor-suppressive functions and promoting proliferation and inhibition of apoptosis.42 Thus, we examined the effects of Klf4 expression in infected HT-29 cells on apoptosis. Our findings suggest that Klf4 expression in infected HT-29 cells did not affect apoptosis. Cells infected with a vaccinia virus that encoded for Klf4-TAT showed significantly lower numbers of apoptotic cells. This correlated well with our findings that GLV-1h391 was significantly less cytotoxic than it parental GLV-1h68 strain and the Klf4-encoding GLV-1h291. It can however be speculated that Klf4 expression can be beneficial in colorectal cancer cells with malignant p53 mutations as the expression of this protein will be reduced. It is, however, of interest to investigate whether Klf4 expression could negatively impact therapy of colorectal cancers with intact p53.
Importantly, a single administration of GLV-1h291 or GLV-1h391 led to significant tumor growth inhibition and overall survival benefits in mice bearing HT-29 tumor xenografts compared to the GLV-1h68 strain. The greatest tumor inhibition was observed in animals treated with Klf4-TAT-expressingrVACV (GLV-1h391). However, comparable virus titers recovered from virus-treated tumors ruled out an exclusively replication-dependent effect on HT-29 tumor growth. Instead, immuno histochemical analysis 21 days post-injection of the virus clearly demonstrated strong Klf4 expression in virus infected tumor patches. Therefore, we suggest that the observed tumor growth inhibition in HT-29 tumors is a consequence of both Klf4-mediated effects and virus replication. By decreasing β-catenin and cyclin D1 levels and thereby slowing cell proliferation, Klf4 expression in tumors may also prevent the tumor from outgrowing the rate of virus replication and tumor oncolysis. The ability of transducible Klf4-TAT to act on yet uninfected neighboring cells might further enhance this effect.
Recently, Lenget al. reported that Klf4 was over expressed in spheroid-cultured, cancer stem cell-like DLD-1 cells, possibly assigning Klf4 a tumor growth promoting role.43 Possible effects on cancer stem cells were not investigated in our study, although we have previously shown that GLV-1h68 replicates preferentially in human breast cancer stem-like cells.44 Notwithstanding these implications, our results support Klf4’srole as a tumor suppressor of colorectal tumors and that klf4 transgene expression can potentially be used as a tool to improve oncolytic virotherapy of colorectal cancers.
In summary, arming ofoncolytic rVACV strains with Klf4 and (transducible) Klf4-TAT caused tumor growth inhibition and significantly improved the survival of animals in the xenograft mouse models of the colorectal cancer cell line HT-29, which otherwise did not respond in vivo to treatment with the oncolytic vaccinia virus GLV-1h68. The results also strongly indicate that the mechanisms underlying the observed therapeutic effects are primarily Klf4-driven. The presented in vitro and in vivo data coincides strongly with the described effects of Klf4 on the Wnt signaling pathway. This indicates that the combination of oncolytic virus activity and Klf4-mediated arrest of cell proliferation enhanced the therapy of colorectal cancer. A systemically administered rVACV encoding, especially, a membrane-permeable Klf4 might provide a powerful tool for the future treatment of colorectal cancer in humans.
Cell and tissue culture
African green monkey fibroblasts (CV-1) and colorectal adeno carcinoma cells (HT-29) were obtained from ATCC (Cat. Nos. CCL-70 & HTB-38, Manass as, VA, USA) and cultured as previously described 24.
Virus constructs
The construction of the recombinant, triple mutant VACV strain GLV-1h68 from the LIVP strain has been previously described.45 Briefly, three expression cassettes encoding Renilla luciferase Aequoria GFP fusion protein, β-galactosidase and β-glucoronidase were inserted into the F14.5L, J2R and A56R loci, respectively, of the viral genome of the LIVP strain. The β-glucuronidase expression cassette in GLV-1h68 was replaced by a Klf4-/Klf4-TAT expression cassette in GLV-1h291 and GLV-1h391 respectively, using guanine phosphoribosyl transferase selection.46 Human Klf4 cDNA was amplified by PCR from the cDNA mix Human Universal QUICK-CloneII™ (Clontech, Mountain View, CA, USA). For the generation of GLV-1h391, the nucleotide sequence 5’-GGTCGCAAGAAACGTCGCC-AACGTCGCCGTCCGCCT-3’ encoding the HIV-1 Tat transduction domain was inserted directly upstream of the Klf4 stop codon by PCR. Both PCR products were cloned into pCRII-Blunt-TOPO® vectors (life Technologies, Carlsbad, CA, USA) and then released by enzymatic PacI/SalI(New England Bio labs, Ipswich, MA, USA) digestion. Subsequently, the cDNA fragments were sub cloned into the vaccinia transfer vectors for the A56R (HA) locus, placing Klf4 and Klf4-TAT under control of the vaccinia synthetic early/late (SEL) promoter. The resulting plasmid constructs pHA-SEL-Klf4 and HA-SEL-Klf4-TAT were sequence confirmed and used for the construction of GLV-1h291 (Klf4) and GLV-1h391 (Klf4-TAT). A description of the genome insertion loci of all three rVACV strains used in this study are presented in Figure 1a.
Viral replication and cell viability/proliferation assays
Viral replication and cell viability assays after infection with GLV-1h68, GLV-1h291 or GLV-1h391 were performed as described previously.47 Data points after infection at MOI 1.0 for all three viruses were extrapolated to calculate the time of infection needed for 50% lethality at the delivered dose. For cell proliferation, HT-29 cells were seeded into 96-well plates at a low density of 1x103 cells /well and cultured at 37°C with conditioned medium from virus-infected CV-1 cells. The XTT Cell Proliferation kit II (Roche, Indianapolis, IN, USA) was used according to manufacturer’s instructions. Absorption was measured at 450 and 700 nm using a Spectra Max microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Preparation of Klf4-containing conditioned media for cell proliferation assays
Confluent CV-1 cells in T225 cell culture flasks were infected with 2x107 plaque forming units (PFU) of either GLV-1h68, GLV-1h291, or GLV-1h391 for 24 h at 37°C. Infected cells were then mechanically harvested and recovered by centrifugation. Cell pellets underwent three freeze-thaw cycles and were then sonicated and treated for 1h at 37°C with benzonase (150 U) (Merck KGaA, Darmstadt, Germany). Suspensions were sterile-filtered using Amicon-15 Ultra tubes (Millipore, Billerica, MA, USA) with a nominal molecular weight limit (NMWL) of 100 kDa. The filter permeates were concentrated using Amicon-15 Ultra tubes (Millipore, Billerica, MA, USA) with a NMWL of 10 kDa to approximately 125-fold volume reduction.
Detection of virus-mediated Klf4 and marker gene expression
Virus-infected HT-29 cells were harvested mechanically, washed with PBS, and collected by centrifugation. Cell pellets were lysed with RIPA, treated with benzonase and protein concentrations were quantified using the DC™ Protein Assay kit (Biorad, Hercules, CA, USA). Samples were then analyzed by Western blotting to determine Klf4 expression or by ELISA (Enzo Lifescience, ADI-900-135, Farmingdale, NY, USA) for β-catenin detection. For Western blot detection, rabbit anti-Klf4 (Abcam, ab72543, Cambridge, MA, USA), rabbit anti-VACV(Abcam, ab35219, Cambridge, MA, USA) or mouse anti-β actin (Abcam, ab97023, Cambrigde, MA, USA) antibodies and HRP-conjugated goat anti-rabbit IgG (Biorad, 170-6515, Hercules, CA, USA) or goat anti-mouse antibodies were used. Immuno reactive bands were visualized using the Opti-4CN detection kit (Biorad, Hercules, CA, USA).
Immunohistological analysis
Infected HT-29 cells were fixed with 3.7% para formaldehyde and permeabilized using 0.5% Triton X-100 (Sigma-Aldrich, St. Louis, MO, USA). Cell nuclei were stained with Hoechst 33258 (Sigma-Aldrich, St. Louis, MO, USA) and virus-mediated GFP expression was used as an indicator for virus infection. Klf4 expression and localization was analyzed using rabbit polyclonal anti-Klf4 antibody (Abcam, ab72543, Cambridge, MA, USA) and Rhodamine-conjugated goat anti-rabbit IgG antibody (Abcam, ab6718, Cambridge, MA, USA). Tumors were surgically excised and prepared as described previously 24. GFP expression was used as an indicator for viral distribution within the tumor tissue. Phalloidin-TRITC (Sigma-Aldrich, St. Louis, MO, USA) was used to label actin. α-Klf4 (Abcam, Cambridge, MA, USA) and 594-conjugated secondary antibodies (Jackson Immuno Research, 111-585-144, West Grove, PA, USA) were used for Klf4 detection.
Quantifying of mRNA transcripts
Total RNA was extracted using the RN easy® kit (QIAGEN, Valencia, CA, USA) and used for first-strand cDNA synthesis with RT Super mix (M-MuLV) w/ oligo(dT)18 (Bio pioneer, San Diego, CA, USA). Quantitative real-time PCR was performed in a Step One Plus Real-Time PCR System (life Technologies, Carlsbad, CA, USA) using fluorescent dye Mesa Green qPCR™ Mastermix Plus for SYBR® Assay kit (Eurogentec, San Diego, CA, USA). Relative quantification was used to quantify gene expression levels in all tested samples. Measurements were conducted in triplicates of each of the three replicate samples. The housekeeping gene 18SRNA was used as an internal control. Primer sequences are listed in (Table S2). Comparative data analysis was done using the CT (2-ΔΔCT) method.48
rVACV strain |
F14.5L |
J2R |
A56R |
GLV-1h68 |
SEL-ruc-gfp |
P7.5-lacZ |
P11-gusA |
GLV-1h291 |
SEL-ruc-gfp |
P7.5-lacZ |
SEL-klf4 |
GLV-1h391 |
SEL-ruc-gfp |
P7.5-lacZ |
SEL-klf4-TAT |
Table S2 Schematic overview of viral constructs
The klf4neg rVACV strain GLV-1h68 encodes gusA (p11 promoter) in the A56R locus. The two klf4pos rVACV strains GLV-1h291 and GLV-1h391 encode for human Klf4 (GLV-1h291) or human Klf4-TAT (GLV-1h391) under the synthetic early/late promoter (SEL) in the A56R locus. P7.5 = VACV 7.5K early/late promoter; P11 = VACV p11 late promoter; SEL = VACV synthetic early/late promoter; ruc-gfp = Renilla luciferase-green fluorescent protein fusion gene; lacZ = beta-galactosidase; gusA = beta-glucuronidase; tfr = transferring receptor; klf4 = Krueppel-like factor 4; TAT – HIV Tat protein transduction domain (GRKKRRQRRRPP)
Analysis of apoptosis
Infected HT-29 cells were washed with PBS and harvested enzymatically with EDTA-Trypsin. Cells were centrifuged 7 min at 1,200 rpm. Cell pellets were re suspended in 2 ml Annexin V binding buffer (BD Biosciences, San Jose, CA, USA) and centrifuged for 7 min at 1,200 rpm. Cell pellets were incubated in 1.5 ml Annexin V binding buffer for 10 min at RT and then centrifuged for 7 min at 1,200 rpm. Cell pellets were then incubated with Annexin V-APC (BD Biosciences, San Jose, CA, USA) and 7-AAD-PerCPCy5 (BD Biosciences, San Jose, CA, USA) for 10 min at RT. Cells were washed with Annexin V binding buffer and centrifuged for 7 min at 1,200 rpm. Cell pellets were re suspended in 150 µL Annexin V binding buffer + 1% para formaldehyde. Samples were acquired using a FACSCan to TM II. Flow data was analyzed using FACS Diva (BD Biosciences, San Jose, CA, USA).
Subcutaneous HT-29 xenografts
Mice were cared for in accordance with approved protocols by the Institutional Animal Care and Use Committee of Explora Bio labs (San Diego Science Center, protocol number EB11-025). Five- to six-week old male Hsd, athymic Nude-Foxn1nu mice (Harlan, Indianapolis, IN, USA) were implanted subcutaneously (s.c.) with 5x106 HT-29 cells (in 100 µL PBS) into the right hind leg. Treatment started when tumors reached a volume of 200-300 mm3. GLV-1h68, GLV-1h291 and GLV-1h391 were administered systemically by intravenous (i.v.) injection of 1x106 PFU in 100 µL PBS at day 0 (n=10 per group)into the lateral tail vein. Control animals (n=5) were injected with PBS only. Absolute tumor growth was measured once a week using a digital caliper and tumor volume was calculated as 0.5 x (height-5 mm) x width x length (mm3). Fractional tumor volume (FTV) (tumor volume on day x / tumor volume on day 0) was used to monitor therapeutic efficacy. Body weight was measured as net body weight (g) (bodyweight (g) – tumor volume/1000 mm3), to exclude tumor mass, and normalized to the initial body weight (day 0). Mice were sacrificed when the net body weight dropped 20% of the initial body weight or the tumor volume exceeded 4000 mm3. The experiment was terminated 49 days post injection (dpi).
Vaccinia virus titers in tumor xenografts
Tumors of virus-treated animals were surgically excised at day 21 post injection and virus titers were determined as described previously 24.
Monitoring of virus-mediatedRuc-GFP expression in live animals
Renilla luciferase signal from live animals was quantified at 14 and 28 dpi after retro orbital (r.o.) injection of 25 µg benzyl-coelenterazine (Nanolight, Pinetop, AZ, USA) / 100 µL PBS. Average mean intensity was determined by layering a fitted region of interest (ROI) over tumor and body, and subtracting the body ROI as background signal from the tumor ROI.
Statistical analysis
The statistical significance of differences between groups of animals was analyzed using one way ANOVA in STATISTICS.). Log rank tests were performed to analyze the statistical significance of survival between the untreated control group and rVACV treatment groups. P< 0.05 was considered significant.
The authors would like to thank Terry Trevino, Jason Aguilar for excellent technical assistance. We thank Dr. Vanessa Cook for providing the pHA-SEL-Klf4 transfer vector. Lastly, we thank Dr. Joseph Capello for critical proof-reading of this manuscript.
This research was supported by Genelux Corporation R&D division. Klaas Ehrig, Lisa Buckel, and Ulrike Donat were recipients of graduate stipends from the University of Würzburg at the time of this research. Jochen Stritzker and Mehmet O. Kilincare salaried employees of Genelux Corporation and have personal financial interest in Genelux Corporation. Aladar A. Szalay is the founder and a shareholder of Genelux Corporation. No competing interests exist for KlaasEhrig, Nanhai G. Chen, Lisa Buckel, and Ulrike Donat.
Klaas Ehrig designed the study, performed and analyzed all experiments, and wrote the manuscript. Nanhai G. Chen participated in conceiving the study and drafting the manuscript. Lisa Buckel participated in drafting the manuscript. JochenStritzker participated in performing animal experiments and drafting the manuscript. Mehmet O. Kilinc participated in conceiving and analyzing flow analysis experiments for apoptosis. Ulrike Donated participated in cloning experiments and drafting the manuscript. Aladar A. Szalay participated in designing the study, writing the manuscript, and gave final approval of submission. All authors read and approved the final version of the manuscript.

©2015 Ehrig, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.