Journal of
eISSN: 2373-6453


Research Article Volume 2 Issue 2
1Department of Child Health School of Medicine, Indonesia
2Department of Public Health School of Medicine, Indonesia
3Faculty of Public Health University of Airlangga Surabaya, Indonesia
4Soerya Maternal and Children Hospital Surabaya, Indonesia
Correspondence: Soegeng Soegijanto, Department of Child Health, University of Airlangga Surabaya, RSIA Soerya Surabaya, Indonesia
Received: December 27, 2014 | Published: March 18, 2015
Citation: Wardana OP, Athiyyah AF, Budiono, Soedirham O, Soegijanto S (2015) The Role of Rotavirus Genotype in the Severity of Acute Diarrhea in Children Under 5 Years Old at Surabaya, Indonesia. J Hum Virol Retrovirol 2(2): 00039. DOI: 10.15406/jhvrv.2015.02.00039
Background: Rotavirus infection is a major cause of diarrhea in children under five years of age in both the developed and developing countries. Rotavirus is often associated with acute infection with high severity level that causes death. The rotavirus genotype, patient immunity, and environmental condition are thought to be related to the severity of the incidence of acute diarrhea due to rotavirus infection in infants and young children.
Objective: To correlate the rotavirus genotypes and the severity degree of acute diarrhea in children under 5 years.
Methods: Cross sectional study was conducted in children aged 1-60 months with acute diarrhea that hospitalized at Gastroenterology ward Dr. Soetomo General Hospital, Surabaya between January to June 2014. Rotavirus examination in stool specimen made from bed-side examination using BD-Rota/Adeno Examine kit, while Rotavirus genotypes examined by molecular methods, namely Reserve Transcription-Polymerase Chain Reaction (RT-PCR) two-steps at the Institute of Tropical Disease (ITD) University of Airlangga. The severity of diarrhea was measured by using a scoring system: Ruuska and Vesikari scores (1990).
Results: A total of 88 children were met the criteria of the study. Of the sample 80.7% were aged 6-24 months. Average value of the total score of severity of diarrhea was 10.21 (SD±2.12). Most Rotavirus genotype G2P4 (19.3%) to group common genotype and genotype G1P4 and G9P4 for group genotyping uncommon with a prevalence of respectively 4.5%. There are significant differences between the types of common genotype and uncommon genotype to the total score of the severity degree of diarrhea (p< 0.05).
Conclusion: The severity degree of acute diarrhea in children with genotype G2P4 is the highest prevalence genotype of Rotavirus infection in Dr. Soetomo General Hospital.
Keywords: Acute diarrhea, Children, Rotavirus genotype, Severity degree
ELISA, Enzyme Linked Immuno Sorbent Assays; RTPCR, Reserve Transcription-Polymerase Chain Reaction
Diarrhea is usually a symptom of an infection in the intestinal tract, which can be caused by a variety of bacterial, viral and parasitic organisms. Infection is spread through contaminated food or drinking-water, or from person-to-person as a result of poor hygiene. Among viruses, rotaviruses are recognized as the single most important cause of severe infantile gastroenteritis worldwide. In the United States, these viruses are estimated to cause between 24.000 and 110.000 hospitalizations in young children annually and 20 to 60 deaths.1 Transmission of rotaviruses occurs by the fecal-oral route, providing a highly efficient mechanism for universal exposure that has circumvented differences in regional and national cultural practices and public health standards. The symptoms associated with rotavirus disease typically are diarrhea and vomiting accompanied by fever, nausea, anorexia, cramping, and malaise that can be mild and of short duration or produce severe dehydration. Severe disease occurs primarily in young children, most commonly between 6 and 24 months of age. Approximately 90 percent of children in both developed and developing countries experience a rotavirus infection by the time they reach 3 years old.1
Rotavirus infection normally provides short-term protection and immunity against subsequent severe illnesses but does not provide lifelong immunity. Furthermore, numerous cases of sequential illness have been reported. Neonates also can experience rotavirus infections, and they occur endemically in some settings but typically are symptomatic. These neonatal infections have been reported to reduce the morbidity associated with a subsequent rotavirus infection.2,3
Rotavirus illnesses also occur in adults and elderly people, but as with other sequential Rotavirus infections, the symptoms usually are mild. Recently, however a series of reports have described a unique association between severe gastrointestinal disease in adults and serotype G2P4 rotaviruses.4 Soenarto et al.5 had studied in 6 hospitals (Jakarta, Palembang, Bandung, Denpasar, Mataram, and Yogyakarta) in 2006 found that 60% children less than 5 years with diarrhea and hospitalized showed positive Rotavirus. The virus also found in 41% diarrhea case that did not hospitalized. Rotavirus infections are a major cause of diarrhea in children under 5 years in both the developed and developing countries. Rotavirus is often associated with acute infection with high severity level that causes death. The virus genotype, patient immunity, and environmental condition thought to be related to the severity of the incidence of acute diarrhea due to rotavirus in infants and young children.2-7
Cross sectional study has been performed in children under five years of age with acute diarrhea and hospitalized at Dr. Soetomo General Hospital from January to June 2014, Based on clinical manifestation and rotavirus examination in stool specimen of the patients, the study found that 88 children met the criteria: 80.7% between the ages of 6 to 24 months, suffered from watery diarrhea and 77.3% of the subject were found as co infection with bacterial.
The result of the study is presented in the following (Table 1-3).
Characteristics |
Number (N) |
Percentage (%) |
Age (month) |
|
|
1 – 5 |
8 |
9.1 |
6-23 |
71 |
80.7 |
24-50 |
9 |
10.2 |
Sex |
|
|
Boys |
51 |
58 |
Girls |
37 |
42 |
Nutritional status |
|
|
Undernourished |
34 |
38.6 |
Normal |
50 |
56.9 |
Overweight |
4 |
4.5 |
Signs and symptoms |
|
|
Watery diarrhea |
88 |
100 |
Vomiting |
80 |
90.9 |
Fever |
|
|
Mild |
14 |
15.9 |
High |
74 |
84.1 |
Dehydration |
|
|
Mild |
78 |
88.6 |
Severe |
10 |
11.4 |
Co infection |
64 |
72.7 |
Table 1 The Characteristics and Clinical manifestation of the 88 Subjects of the Study
Variation type |
Number |
Percentage (%) |
Type G |
||
G1 |
26 |
29.5 |
G2 |
28 |
31.8 |
G3 |
2 |
2.3 |
G4 |
4 |
4.6 |
Subtotal |
60 |
68.2 |
Type P |
||
P[4] |
28 |
31.8 |
P[8] |
23 |
26.1 |
P[6] |
24 |
27.3 |
Subtotal |
75 |
85.2 |
Table 2 Variation Type Gen VP7 (Type G) and Type Gen VP4 (Type P) rotavirus of 88 cases
Table 2 presents the variation of common genotype shows that type G2 and type P [4] are the most common found in this study.
Common genotype |
Number |
Percentage (%) |
G1P[8] |
10 |
11.4 |
G1P[6] |
11 |
12.5 |
G2P[4] |
17 |
19.3 |
G2P[6] |
3 |
3.4 |
G3P[6] |
2 |
2.3 |
G4P[6] |
2 |
2.3 |
G4P[8] |
1 |
1.1 |
Subtotal |
46 |
52.3 |
Table 3 The Variation Genotype Combination of type G-Common and type P-Common
Table 3 present the Variation genotype that most found in the study especially for G2P[4] was the highest number.
Diarrhea due to rotavirus infection occurs in early childhood in developing countries and in developed countries2-4,6 It has been estimated that, from 1986 to 1999, a median of 22% range 17-28% of acute diarrhea cases in children less than five years of age were due to rotavirus, but this proportion has nearly doubled recently from 2000 to 2004 to become 39% range 29-45%.4-6 Incidence of deaths due to rotavirus diarrhea in developing countries the estimated average is 1:25. On the other hand, in developed countries, hospitalization due to rotavirus in children under five years of age the estimated is between 1:20 and 1:80.5-7
Soenarto et al.8 that performed diarrhea disease surveillance in Yogyakarta hospital found that 38% of stool sample showed, positive Rotavirus particle with diarrhea. Meanwhile, Corwin et al.9 examined the stool of children with diarrhea who hospitalized in Kupang, East Nusa Tenggara, using Enzyme linked immuno sorbent assays (Elisa) and Reserve Transcription-Polymerase Chain Reaction (RT-PCR), found 48% sample showed positive Rotavirus. More recently, Putnam et al.10 found 45.5% children with diarrhea who hospitalized in Denpasar, Jakarta and Makasar showed a positive Rotavirus [5,6,10]. Soenarto et al. [8] performed a study in 6 hospitals (Jakarta, Palembang, Bandung, Denpasar, Mataram, and Yogyakarta) on 2006 found that 60% children less than five years of age with diarrhea and hospitalized showed positive rotavirus. The virus also found in 41% diarrhea case who did not hospitalized.6,10
The serotype is the most important antigenic determinant of Rotavirus and is defined traditionally by serological assays. In the later, a number for P genotype is designated within a squared bracket. The serotype of prototype human rotavirus strain is described as G1P1A.8 There are currently 16 G serotype and 26P serotype described in the literature, but the G and P type combination detected in human rotavirus are mostly limited to G1P[8], G2P[4], G3P[8], G4P[8], and G9P[8].11,12
G and P type combination in this study found that the highest prevalence type G2 type P [4] (Table 1).
All of showed a severity clinical manifestation of rotavirus diarrhea infection. The pathogenesis of Rotavirus diarrhea is complex and incompletely understood especially with potential roles of viral enterotoxin, mal absorption related to mucosal by the enteric nervous system (ENS). Postmortem examination of the gastrointestinal tract of gnotobiotic pigs with diarrhea after experimental infection with a virulent human rotavirus strain demonstrated that virus replicates primarily in the villous epithelium of the small intestine.13,14 This pattern is consistent with the patchy villous epithelial distribution of rotavirus antigen noted after immuno fluorescent staining of duodenal biopsy specimens from children with severe gastroenteritis. Light microscopic examination of such duodenal biopsy specimens reveals shortened and blunted villi with a cuboidal epithelium, crypt hypertrophy, and monocular cell infiltration of the lamina propria.13-16
The severity of diarrhea in children with rotavirus gastroenteritis correlates with the degree of mucosal damage, which suggests that mal absorption related to loss of absorptive cell may contribute to rotavirus diarrhea late in infection.13-16 However, in experimentally infected gnotobiotic pigs, diarrhea precedes villous atrophy. Similarly, small intestinal biopsies from children with relatively mild rotavirus gastroenteritis do not consistently display histological changes, probably reflecting patchy epithelial injury (Figure 1)14,15,17
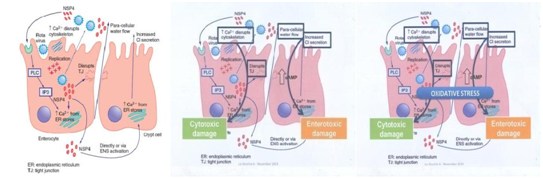
Figure 1 Mechanisms of Pathogenesis Rotavirus.16
Infants and young children with diarrhea caused by rotavirus are more likely to have severe symptoms and become dehydrated than patients with diarrhea related to other common enteric pathogens.18-20 The clinical features of rotavirus gastroenteritis in humans have been studied in experimental infections in adult. In one such, 4 of 18 adult volunteers develop vomiting 1 to 3 day after oral administration of a virulent rotavirus strain.21 This was followed by diarrhea lasting 1 to 4 days and associated with anorexia, crampy abdominal pain, and low grade fever. Viral shedding in stool was detected for 6 to 10 or more days. Two thirds of the adult volunteers developed serologic evidence of infection without disease. Similarly, most natural rotavirus infection of adult is asymptomatic, manifested only by a rise in antibody titer. However, natural rotavirus infection of adults can also causes severe and even fatal disease.22,23
Clinical manifestation of our study, 88 cases of children with acute rotavirus diarrhea showed: watery diarrhea 100%, vomiting 90.9%, high grade fever 84% and mild grade fever 15.9%, mild dehydration 88.6% and severe dehydration 11.36%, and co-infection with other bacterial 72.7%. A limited number of serotypic combinations cause the majority of symptomatic infections of humans. Rotaviruses with four such combinations G1P [8], G2P [4], G3P [8], and G4P [8], caused 88.5% of decades from 1973 to 2003.11,12 However there is substantial geographic and temporal variation in strain distribution. For, example GIP [8] causes over 70% of rotavirus infection in North America and Europe but only 23% in Africa. Serotype and genotype diversity is greater in Africa, Asia, and South America, Europe, and Australia.
Rotavirus gastroenteritis is not clearly distinguished from other causes of acute gastroenteritis on clinical grounds alone. Because the standard treatment for rotavirus gastroenteritis is rehydration and supportive care, a specific microbiologic diagnostic is not required in most cases. However, with prolonged diarrhea in complicated cases, in immune compromised hosts, when alternative diagnoses are considered, or when epidemiologic or infection control date are needed, it may be desirable to establish rotavirus as the etiologic agent. Definitively diagnosing rotavirus gastroenteritis may also to prevent unnecessary and potentially harmful use of antibiotics.
Rotavirus can be detected by numerous techniques, including variety of commercial antigen: assay, RT-PCR, electron microscope, immune electron microscope, polyacrylamide gel electrophoresis (PAGE) for viral genomic RNA, and viral culture.24,25 Detection of viral antigen in stool or rectal swabs, most commonly using enzyme-linked immuno sorbent assay (ELISA) or latex agglutination formats, forms the basis for practical, commercially available, and widely used diagnostic kits. Latex agglutination is particularly suitable for use in areas with limited resources, although a confirmatory technique is desirable to evaluate indeterminate result because of the limited sensitivity of the test. Commercial antigenic assay primarily detect the VP2 and VP6 proteins of the sub viral double-layered particle and detect only group A rotaviruses.24,26 Serotype-specific ELISAs, based on recognition of VP7 or VP4, allow determination of serotype without the need to perform neutralization assay. Although there are various techniques for measuring serum, fecal, and salivary antibodies against rotavirus, the acute and generally self-limited nature of rotavirus infection limits the usefulness of these techniques for clinical decision making.
Multiplexed RT-PCR has become a major diagnostic technique used in epidemiologic studies. RT-PCR allows determination of P and G types and permits finer definition of strain differences. Real-time RT-PCR provides greater sensitivity and speed than conventional or nested PCR diagnostic.24,26 DNA oligo nucleotide microarray methods now being introduced offer greater robustness to sequence drift (which can prevent PCR amplification if a primer binding site is affected) and greater ability to distinguish a mixed infection from a single strain infection.23,25,27
Recommendation for treatment is summarized in a guideline from the Centers for Disease Control and Prevention (CDC). As rotaviruses gastroenteritis is generally self-limited and dehydration is the primary cause of morbidity, rehydration and restoration of electrolyte balance are the primary therapies. Oral rehydration solutions (ORSs) are effective in treating dehydration related to rotavirus gastroenteritis, even in the presence of moderate vomiting, and are preferred over IV rehydration in cases of mild or moderate dehydration. The principle on which these solutions are based is the solute-coupled co transport of sodium by enterocytes, which continues to operate even in the damaged gut; Effective solutes include glucose, amino acids, and short oligopeptides.
Diosmetic; Adsorbent clay, Alumino-magnesium silicate, Binds to endo- and exotoxins , Binds bacteria, and rotavirus, Modifies physical properties of gastric mucus.18
Because lack of access to treatment is one of the major causes of childhood mortality from rotavirus, and improved sanitation has limited impact on rotavirus prevalence, prevention by immunization is a critical approach to decreasing the impact of this infection.28 In 1998, the first human rotavirus vaccine, Rota shield was approved by the US Food AND Drug Administration (FDA) and was recommended for infants by the Advisory Committee on Immunization Practices (ACID).29,30 This quadrivalent, reassortants vaccine was based on a modified “Jennerian” approach, using a live animal virus to immunized humans.24,26,29 To broaden the serotype specificity of the immune response, VP7 from each of the G types 1 to 4 was presented on the genetic background of a simian rotavirus strain (RRV), which is attenuated for humans on the basis of host range restriction and passage in cell culture.28,31 In phase III trials, Rota Shield proved safe and highly effective against moderate to severe diarrhea in both developed (United States and Finland) and developing (Venezuela) countries.31
Since virtually all children will have experienced rotavirus infection by the age of 3-5 years in both developing and develop countries, it is clear that the high standards of hygiene and sanitation practiced in developed countries are not sufficient to prevent the spread of rotavirus infections within of community, Thus prophylaxis of severe rotavirus gastroenteritis by vaccine remains as the only practical preventive measure.28,32 The first licensed rotavirus vaccine, a rhesus monkey rotavirus-based tetravalent human reassortants vaccine (Rota shield), was withdrawn after this live, oral vaccine was associated with the development of intestinal intussusceptions in approximately 1:10.000 vaccine recipients in USA. Two new rotavirus vaccines, Rotarix, have recently completed phase III clinical trials, each involving more than 60.000 infants. Both vaccine were found to be safe when given to infants under 3 months of age and were >85 % efficacious in preventing severe gastroenteritis due to rotavirus.29,30,33 Rotarix is a monovalent human rotavirus vaccine of serotype GIP1A [8], whereas Rotateq is a pentavalent bovine-human reassortants vaccine comprising types G1, G2, G3, G4 and P [8].
Severity degree of acute diarrhea in children under 5 years old in this study mostly caused by the combination with genotype G2P [4]. This result seemed similar to the other study elsewhere.11,12 Although rotavirus gastroenteritis is generally self-limited but as dehydration is the primary cause of morbidity, rehydration and restoration of electrolyte balance are the primary therapies.
But the prevetive measure should be the most recommended such as:
None.
None.

©2015 Wardana, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.