Journal of
eISSN: 2373-6453


Review Article Volume 11 Issue 1
1M.D. Obstt & Gynae, specialist reproductive endocrinology & Infertility specialist, Centre for Human Reproduction, India
2D.N.B, Scientific Director, Ex-Rotunda-A Centre for Human Reproduction, India
3M.D. DM. (Std) (Neurology) Consultant Neurologist, Swami Satyanand Hospital, India
Correspondence: Dr. Kulvinder Kochar Kaur, M.D (Obstt & Gynae, specialist reproductive endocrinology & Infertility specialist), Scientific Director cum Owner, Centre for Human Reproduction, 721,G.T.B. Nagar, Jalandhar-144001, Punjab, India, Tel 91-181-9501358180, 91-181-4613422, Fax 91-181-4613422
Received: January 11, 2024 | Published: January 25, 2024
Citation: Kulvinder KK, Gautam NA, Mandeep S. Targeting dysfunctional mitochondrial metabolism of hepatocytes caused hepatitis B virus (HBV) in the treatment of the chronic HBV infection- a narrative review. J Hum Virol Retrovirol. 2024;11(1):4-12. DOI: 10.15406/jhvrv.2024.11.00273
Mitochondria possess a significant part in generation of adenosine triphosphate (ATP), Reactive oxygen species (ROS), in addition to the controlling of the innate immune reactions along with apoptosis. Numerous viruses interfere with the mitochondrial actions for facilitating their replication along with result in cell injury. Hepatitis B virus(HBV) portrays a hepatotropic virus which possesses the capacity of resulting in robust liver diseases inclusive of cirrhosis in addition to Hepatocellular carcinoma(HCC).This virus further possesses the capacity of changing the mitochondrial working in addition to metabolism for facilitating its replication along with their continuation. Having earlier reviewed the part of generation besides the epigenetic controlling of the ccc DNA micro chromosome, the manner host as well as viral factors impact transcription besides if utilization of epigenome editing could be done for silencing HBV ccc DNA forever and why persistence of HBV takes place besides mitochondrial metabolism, mitophagy in ageing and role in fatty acid metabolism here we have concentrated on Hepatitis B virus(HBV) along with described the recent advancements in our acquisition of knowledge regarding the association amongst HBV in addition to mitochondrial metabolism. Here we conducted a narrative review utilizing search engine pubmed, Google scholar; web of science; embase; Cochrane review library utilizing the MeSH terms like Hepatitis B virus; mitochondrial metabolism; mitophagy; CD8+T cells; oxidative phosphorylation (OXPHOS);viral replication; viral persistence. We have detailed the recent advancements in the crosstalk HBV as well as mitochondrial metabolism in addition to its actions on HBV replication of addition to persistence as well as how utilization of this knowledge can help in treatment of HBV-add of before chronic Hepatitis B (CHB) infection.
Keywords: hepatitis b virus (HBV) infection, mitochondrial metabolism, viral replication, persistence, oxidative phosphorylation
OXPHOS, oxidative phosphorylation; TCA alias Kreb’s cycle, tricarboxylic acid cycle; FA, fatty acids; ROS, reactive oxygen species; IMM, inner mitochondrial membrane; NADPH, nicotinamide adenine nucleotide phosphate reduced form; FADH2, flavin adenine nucleotide phosphate; ETC, electron transport chain; ATP, adenosine triphosphate; ZIKV, zika virus; HIV1, Human immunodeficiency virus type 1; HCV, Hepatitis C virus; HBsAg, Hepatitis B surface antigen; HCC, Hepatocellular carcinoma; HBx, HBV protein x; HBeAg, e antigen; RC-DNA, relaxed circular genomic DNA; ccc DNA, covalently closed circular DNA; pgRNA, pregenomic RNA; ALT, alanine amino transferase; LCFAs, long chain CoA fatty acids; G3P, glycerol-3 –phosphate; cGDDH/ GPD1, cytosolic G3P dehydrogenase; mGDDH/ GPD2, mitochondrial FAD- G3P dehydrogenase; VDAC, voltage dependent anion channel; COXIII, cytochrome c oxidase subunit III; NFκB, nuclear factor κB; COX2, cyclooxygenase2; RIG, retinoic acid inducible gene; RLRs, like receptors; PRRs, pattern recognition receptors; MAVS, mitochondrial antiviral signalling; IFN, type1 interferon; HK, hexokinase; LDHA, lactate dehydrogenase; STATs, signal transducers and activators of transcription; SOCS3, Suppressor of cytokines signaling 3; Nrf2, nuclear factor erythroid-2-related factor-2; DNMT, DNA methyltransferases; HDACs, histone deacetylales; MT-CO III, mitochondrially encoded to cytochrome c oxidase III; PTEN, gene phosphatase ,tension homolog; PINK1, induced kinase; LC3B, the microtubule correlated protein 1A/1B light chain 3B; ER, endoplasmic reticulum stress; MMP, mitochondrial membrane potential; CTL, CD8+cytotoxic T lymphocytes; PD-1, programmed cell death; PARP1, poly ADP ribose polymerase 1; PARylation, poly ADP ribosylation; DDR, DNA damage response; ACAT, acylCoA: cholesterol-acetyltransferase; LDs, lipid droplet; TNF-α, tumor necrosis factor alpha; IL-6, Interleukin; MRC1, mannose receptor C type1; PBMCs, peripheral blood mononuclear cells; IT, immune tolerance; IA, immune activation; IC, inactive carrier; ENEG, HBeAg-negative hepatitis; MTA, mitochondria-targeted antioxidants; EO, eosinophil; RDW-CV, count ,coefficient of variation of red blood cell distribution width; NLRP3, nucleotide-binding domain, leucine-rich-repeat containing family, pyrin domain-containing inflammasome; TOM40, translocases of OMM; Ripk2, channels, receptor interacting protein kinase 2; IAV, influenza a virus; ssRNA40, HIV long terminal repeat region; T1/ T2DM, type 1&2 diabetes mellitus
Mitochondria portray intracellular organelles which are key for the generation of the energy, Calcium (Ca2+) homeostasis as well as cell signaling. They possess key part in antiviral reactions in addition to numerous viruses have been acknowledged to modulate the mitochondrial working for escalating their replication as well as survival.1,2 Mitochondria are further implicated in a plethora of metabolic pathways, inclusive of oxidative phosphorylation (OXPHOS), tricarboxylic acid cycle (TCA alias Kreb’s cycle), β oxidation for the transformation of fatty acids (FA) into acetyl CoA for it gaining entry to the TCA cycle ;the generation of the reactive oxygen species (ROS), in addition to controlling apoptosis (Figure 1).3
OXPHOS implicates the shifting of the electrons via a series of complexes proteins having placement in the inner mitochondrial membrane (IMM), which leads to the generation of the adenosine triphosphate (ATP) - the primary source of cellular energy (Figure 1). TCA cycle portrays the critical aspect of the aerobic respiration.It forms the nicotinamide adenine nucleotide phosphate (NADPH) in addition to flavin adenine nucleotide phosphate (FADH 2) with regards to fuelling the electron transport chain (ETC), for the generation of ATP. Viruses possess the capacity of resulting in disturbance of mitochondrial metabolism that leads to injury to cells to facilitate their replication. Zika virus (ZIKV) possess the capacity of NAD+- correlated metabolic pathways, inclusive of downregulation of TCA as well as OXPHOS in neurons along with neuroblasts that might result in microcephaly generation in the mouse brain.4 Human immunodeficiency virus type 1 (HIV1) has further been illustrated to change the metabolism of the CD4+ T cells by escalating OXPHOS for facilitating its replication.5 Hepatitis C virus (HCV) core protein might further have placement in the outer mitochondrial membrane (OMM) leading to hampering of the mitochondrial electron transport in addition to escalated formation of the ROS.6
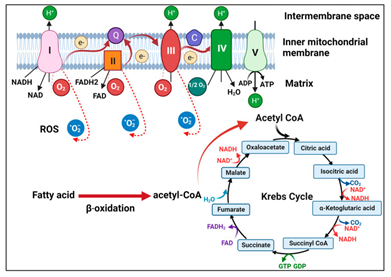
Figure 1 Courtesy ref no-3-Illustration of mitochondrial metabolism. Fatty acids undergo β-oxidation to generate acetyl-CoA, which then drives the TCA cycle in the matrix of mitochondria. NADH and FADH2 generated by the TCA cycle donate electrons to complex I and complex II, respectively, on the inner mitochondrial membrane. The electrons are then transferred through ubiquinone (Q) to complex III and then via cytochrome C (C) to complex IV. The electrons are then transferred to the oxygen molecule to form water. During this process, complexes I, III, and IV pump protons to the intermembrane space to generate a proton gradient to drive ATP synthesis by ATP synthase (complex V). During OXPHOS, electrons may also leak and interact with oxygen to form superoxide (i.e., RSO).
HCV has been illustrated to stimulate the induction of dynamin -related protein (Drp1) modulated - mitochondrial fission along with mitophagy akin to selective elimination of mitochondria by autophagy for ensuring the continuation of the replication,7 as well as the repression of this event decreases glycolysis, generation of ATP as well as viral liberation.8 This changed outcome of different inclusive of stimulation of the metabolic switch towards glycolysis that possesses the capacity of formation of acetyl-coA ,an intermediate in the generation of the triglycerides (TG) .This results in the generation of lipid droplet (LDs) as well as diminished FA β oxidation.9
Existence of other viruses is further there which possess the capacity of changing the mitochondrial metabolism of the cells.
Earlier we had reviewed the part of CRISPR/ Cas9 system regarding treatment of HBV as well as role in treatment of other diseases, besides role of epigenetic treatments for Diabetic Kidney Disease (DKD), along with in placental dysfunction ,along with reviewed the generation besides the epigenetic controlling of the ccc DNA micro chromosome, the manner host as well as viral factors impact transcription besides if utilization of epigenome editing could be done for silencing HBV ccc DNA forever & why persistence of HBV takes place,10–14 besides mitochondrial metabolism, mitophagy in ageing15,16 & role in fatty acid metabolism.17 However in this review we have concentrated on mitochondrial metabolism in Hepatitis B virus (HBV) along with described the recent advancements in our acquisition of in the association amongst HBV in addition to mitochondrial metabolism.
Here we conducted a narrative review utilizing search engine pubmed, google scholar ;web of science; embase; Cochrane review library utilizing the MeSH terms like Hepatitis B virus; mitochondrial metabolism; mitophagy; CD8+T cells; oxidative phosphorylation (OXPHOS); viral replication; viral persistence; Epigenetics; Histone Epigenetic modifications from 1970 to 2024 January till date.
We found a total of 300 articles out of which we selected 75 articles for this review. No meta-analysis was done.
Life cycle of HBV
HBV represents one of the considerably significant human pathogens. It possesses the capacity of resulting in robust liver diseases inclusive of acute as well as chronic hepatitis, cirrhosis in addition to Hepatocellular carcinoma (HCC). HBV is implicated in chronic infection in around 300 million individuals resulting in determined 820,000 deaths yearly.18 Acquisition of virus in the maximum chronic HBV patients takes place from their mothers early in life. Compared to mother- child transmission alias vertical transmission, horizontal transmission amongst non-relatives (for instance via sharing of needles for injection of drugs or through sexual actions) generally results in self-restricted acute infection.19 HBV is not involved in resulting in lytic infection in addition to there is existence of myriad of asymptomatic HBV carriers. HBV stimulated liver damage might implicate the HBV variants appearing as well as is usually correlated with the activation of host CD8+T cells reactions that are dysfunctional at the time of chronic HBV infection.20
HBV portrays a small DNA virus belonging to the hepadna viruses’ family. It possesses a considerably small host varieties infecting just humans along with occasional primate species (spp).21 Dane et al.,22 in 1970 saw these viral particles with the utilization of an electron microscope, displaying the existence of 3 kinds of particles in the serum of the infected subjects:
i) Spherical particles having a diameter of 42nm,
ii) Spherical particles having a diameter of 22nm in addition to
iii) Particles having a diameter of 22nm.13,4
The 42nm has been subsequently referred to as the Dane particles, that represent mature virions comprised of a lipid envelope in addition to 3co-carboxy terminal viral envelope proteins-alias large (L), middle (M) as well as small (S) Hepatitis B surface antigen (HBsAg). Furthermore, the Dane particles reveal the core antigenic estimator referred to as the core antigen (HBc). The main protein comprising the core particle is the core protein that generates a protein shell which surrounds the viral DNA genome. Recent studies have suggested that greater than 90% of the 42nm do not possess HBV DNA genome.20,23 The HBV genome portrays a circular along with partly double stranded DNA molecule having a length of about 3.2 kilobases. Four genes get encoded by it; C, P, S as well as X.
i) The C gene encodes core protein in addition to an associated protein alias pre core protein;
ii) P gene encodes for the viral DNA polymerase that is further a reverse transcriptase;
iii) The S gene encodes for the 3 HBsAg proteins;
iv) The X gene encodes for the HBV protein x (HBx).
The pre core protein is the precursor of the e antigen (HBeAg) observed in the serum of the HBV patients. Compared to Dane particles, that possess a core, 22nm particles portray empty enveloped particles. Besides Dane particles as well as 22nm particles, naked core particles are further liberated from HBV infected cells.24
HBV portrays a hepatotropic virus gaining entry into the hepatocytes through a crosstalk with its receptor sodium-taurocholate-co transporting peptide (NTCP) present on the cell surface.25 On being within the cell administration of the relaxed circular genomic DNA (RC-DNA) by the core protein into the nucleus, where its transformation takes place to covalently closed circular DNA (ccc DNA).This ccc DNA works in the form of a transcription template for all of the viral mRNAs. The core protein mRNA possesses larger size in contrast to the genome as well as is referred to as pregenomic RNA (pgRNA). It further encodes for the viral DNA polymerase. On the generation of the core protein, packaging of the pgRNA is done to generate a core particle, where pgRNA gets reverse transcribed in addition to transformed into rc DNA by the viral DNA polymerase which further gets packaged.20 The core particles possessing the rc DNA might crosstalk with HBsAg in intracellular membranes to generate mature viral particles to be liberated from HBV infected hepatocytes. Or in the form of an alternate mode rc DNA might be transported back to the nucleus to repair in addition to amplify the ccc DNA pool. HBV DNA might be further incorporated into the host chromosomes. Despite, the incorporation of HBV DNA might further guide the generation of the viral transcripts, this incorporation is not an imperative step in the viral life cycle.26
HBV along with host metabolism
HBV infection possesses the capacity of resulting in significant metabolic alterations in the patients with HBV. An untargeted metabolomics assessment performed by Yu et al.,27 in serum samples of 199 HBsAg positive subjects possessing active (for instance serum HBV DNA >100IU/ml) as well as inactive (for instance serum HBV DNA <100IU/ml) HBV replication in addition to variation of the degree of liver diseases inclusive of chronic hepatitis, cirrhosis in addition to HCC, significant changes in metabolites inclusive of diminished amino acids along with escalation of phosphatidyl cholines were found in the patients having active HBV replication.27
In case of another targeted metabolomics assessment of serum samples significant metabolic changes inclusive of escalation of free fatty acids (FFA), acyl carnitine as well as plasmalogens in addition to reduction of the phospholipids (PL’s), sphingomyelins, triglycerides(TG) in the phase of immune tolerance in case of HBV infection took place (for instance HBsAg as well as HBeAg positive having a mean DNA copy number of 8.82 log in addition to serum alanine amino transferase (ALT) quantities<40 were further observed.28 An evaluation of adenosine triphosphate (ATP) quantities in serum further displayed significant reduction of the ATP quantities in the patients with HBV in contrast to the healthy controls probably in view of dysfunctional mitochondrial working in patients with HBV.29 These observations pointed that HBV might impact the metabolic pathways in HBV.
Actions of the HBV on the metabolism of hepatocytes
HBV possesses the capacity of resulting in alterations of the metabolism of hepatocytes.30 Utilization of an adenoviral vector with regards to administration of an over length HBV genome into rat hepatocytes, that worked in the form of a surrogate model for the infected human hepatocytes, it was observed that HBV possessed the capacity of changing metabolome of hepatocytes. Further transcriptomic evaluation by Lambotagne et al.,31 displayed changes of metabolic pathways which were inclusive of long chain CoA fatty acids (LCFAs) metabolism, glycolysis as well as glycogen metabolism.31 The glycerol-3–phosphate (G3P) shuttle portrays a pathway which results in the translocation of the electrons generated at the time of glycolysis across the IMM for the OXPHOS. Its modulation takes place by the combination of actions of cytosolic G3P dehydrogenase (cGDDH/ GPD1) along with FAD- G3P dehydrogenase (mGDDH/ GPD2). An evaluation of the crosstalk amongst HBV in addition to G3P shuttle observed that GPD2; however not GPD1 repressed HBV replication by the enrolment of the ubiquitin ligase TRIM28 for the generation of a complex with HBx, a controlling protein of HBV leading to the proteasomal breakdown of the HBx.32 There is no requirement of actions of GPD2 for diminishing HBx which probably is a host defense mode against HBV. This might be further the cause of HBV diminishing the quantities of GPD2 in case of liver tissue.32 This diminishing of the quantities of GPD2 by HBV probably impacts the G3P pathway which might reason out the diminished glycerophospholipids in addition to escalated plasmalogens observed in the serum of the chronic HBV subjects.28
Part of the HBx on the mitochondrial metabolism of hepatocytes
The HBx protein of the HBV describes a controlling protein possessing a plethora of functions. It has the features of activating transcription factors, controlling signaling pathway, of facilitating hepatocarcinogenesis in addition to that of greater significance gene expression.20,33 Its placement might be in the mitochondria, through numerous areas of sequence.34 It possesses the capacity of cross talking with the voltage dependent anion channel(VDAC),35 a channel spanning across the mitochondrial outer membrane in addition to cytochrome c oxidase subunit III(COXIII), a protein spanning across the mitochondrial inner membrane in addition to a subunit of respiratory complex IV.36 HBx further possesses the capacity of activating transcription factor nuclear factor κB(NFκB) for avoidance of the depolarization of the mitochondrial membranes. Nevertheless, once NFκB is hampered, HBx would stimulate the depolarization of the mitochondrial membranes.34 HBx further possesses the capacity of the induction of the expression of cyclooxygenase 2 (COX2) via stimulating the induction of the ROS for facilitating the cell growth.37
Compared to MARCH5 one more E3 ubiquitin ligase having placement on the outer aspect of mitochondrial membrane might further crosstalk through its N-terminal Ring domain with HBx for the modulation of the breakdown of the HBx.38 (Figure 1).This results in repression of HBx stimulated ROS generation, mitophagy as well as COX2 gene expression.
HBx, has been further illustrated to be binding of Orail protein which describes a store operated calcium entry constituent on the plasma membrane (PM) for the escalation of intracellular calcium quantities, through an influx of extracellular calcium.39 This influx results in escalated calcium uptake by VDAC into mitochondria as well as calcium signaling pathways (Figure 2).40
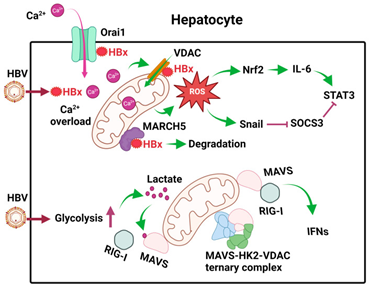
Figure 2 Courtesy ref no-3-Effects of HBV on mitochondrial metabolism in hepatocytes. After the infection of hepatocytes, HBV expresses its HBx protein, which binds to Orai1 to promote the uptake of Ca2+, which then enters mitochondria to cause calcium overload and activate calcium signaling. HBx also binds to VDAC to induce ROS. MARCH5, an E3 ubiquitin ligase, binds to HBx to promote its degradation. HBV also induces glycolysis in hepatocytes to generate lactate, which binds to MAVS on mitochondrial membranes to disrupt RIG-I signaling. In addition, HBV also promotes the formation of the MAVS-HK2-VDAC ternary complex to prevent MAVS from interacting with RIG-I. The ROS produced by mitochondria induces the expression of Nrf2 to induce the expression of IL-6 and activate STAT3. It also induces the expression of Snail to inhibit the expression of SOCS3, an inhibitor of STAT3. These effects of ROS lead to constitutive activation of STAT3 and cell proliferation.
Thereby HBx impacts mitochondrial working in myriad manners for ensuring mitochondrial impairment.41
Part of the mitochondria in innate immune reactions against HBV
Cellular metabolism is commonly associated with cellular innate immune reactions, retinoic acid inducible gene (RIG)-like receptors (RLRs) which are inclusive of RIG in addition to MD5 portray pattern recognition receptors (PRRs) which get activated by pathogen –associated molecular patterns (PAMP) which are existent on RNA molecules. On their activation binding takes place to the signaling adapter referred to mitochondrial antiviral signaling (MAVS) on mitochondrial membranes for the activation of downstream signaling for the activation of type1 interferon (IFN) as well as pro-inflammatory cytokines. It has been illustrated recently that HBV facilitated glycolysis for the repression of RIG-I modulated IFN induction.42 In this exquisite study it was displayed that primary human hepatocytes (PHH) which were infected by HBV possessed escalated quantities of metabolic intermediates for instance pyruvate, lactate in addition to other metabolic intermediates of the TCA cycle. The escalation of lactate was in view of activation of hexokinase (HK) as well as lactate dehydrogenase (LDHA) by HBV. Subsequently binding of lactate with MAVS takes place for the avoidance of its crosstalk with the RIG present on mitochondrial membranes (Figure 2).
Additionally, HBV further segregated MAVS by facilitating the production of ternary complex constituted of the MAVS, HK along with VDAC present on mitochondrial membranes (Figure 2). These actions of the HBV on MAVS disturbed its downstream signaling with the following IFN induction as well as escalated viral replication.42 These studies have pointed that HBV possesses the capacity of modulating cellular metabolism for evasion of the innate immune reactions for the advantages of their replication.
HBV further stimulated expression of the IL6, which causes after activation of signal transducers and activators of transcription (STATs), for facilitating hepatocellular proliferation, in liver tumor cell lines along with the liver tissues of the HBV patients.43 IL6 expression stimulation took place in view of ROS induction in the mitochondria by HBV, since it got ameliorated on the treatment of the cells with the utilization of rotenone resulting in blockade of ROS formation in mitochondria. ROS results in the upregulation of the transcription factor nuclear factor erythroid-2-related factor-2 (Nrf2) that further causes stimulation of the IL6. Suppressor of cytokines signaling 3 (SOCS3) portrays a negative feedback controller of the IL6/ STAT3 signaling pathway.
Intriguingly, blockade of the expression of the SOCS3 further was caused by ROS that stimulated the expression of the transcription factor Snail. Binding of the Snail takes place with the E boxes of the SOCS3 promoter that modulated the epigenetic silencing of SOCS3 in correlation with DNA methyltransferases (DNMT) in addition to histone deacetylates (HDACs). SOCS3 silencing resulted in the sustenance of the activation of IL6/ STAT3 pathway that might be plausibly implicated in the generation of the HBV induced hepatocarcinogenesis (Figure 2).43
Incorporation of the HBV DNA into mitochondrial DNA
HBV DNA possesses the capacity of getting incorporated into the host chromosome with the incorporation might result in activation/ interference with the cellular genes for facilitating hepatocarcinogenesis.44
Intriguingly, a recent study further illustrated that the HBV DNA possesses the capacity of getting incorporated into the mitochondrial DNA in the OXPHOS genes in addition to the D-loop area whose localization is in the non-coding area of the mitochondrial DNA.45 Enrichment of the HBV DNA breakpoints preS/ S area of the viral genome when it got incorporated into the D-loop as well as in the X gene (inclusive of its upstream enhancer I/ X promoter in addition to the downstream pre core sequence) once it got incorporated into the mitochondrial protein sequence.45 The incorporation of the HBV DNA which traverses X as well as procure sequence in mitochondrial encoded to cytochrome c oxidase III (MT-CO III ) gene was further displayed in one more study by Oikawa et al.46 In the Oikawa et al.,46 study HBV DNA in addition to host DNA at the juncture of the incorporation were observed to be hyper methylated. No clarification is present with regards to the manner incorporation of the HBV DNA takes place in the mitochondrial genome. Nevertheless, in view of the observations of the HBV RNA sequences in mitochondria as well, it got pointed that the incorporation of the HBV DNA takes place by HBV RNA plausibly.45 No clarification is present with regards to the biological importance of the incorporation of the HBV DNA, despite it pointing that in the HBV might be plausibly controlling mitochondrial metabolism through the disturbance of the mitochondrial genome.
HBV along with mitophagy
HBV might be further influencing mitochondrial metabolism through the induction of mitophagy, i.e., the selective elimination of mitochondria by autophagy. Kim et al.,48 displayed that the HBV or its HBx protein might possesses the capacity of induction of the perinuclear aggregates of mitochondria in addition to the residing of the Drp1 in the mitochondria by stimulating its phosphorylation at serine-616.48 The residing of the Drp1 in the mitochondria possesses the capacity of stimulating fission,48 causing separation of the mitochondria which are depolarized, that represent the targets for the depletion by mitophagy.49 Noticeably, induction of the mitochondrial aggregates besides taking place in case of HBV further takes place with the other viruses for instance rubella virus50 along with respiratory syncytial virus.51 As per Kim et al.,47 apart from resulting in induction of the mitochondrial fragmentation, HBV or its HBx protein further had the capacity of induction of the expression of phosphatase as well as tension homolog (PTEN) induced kinase [PINK1], Parkin in addition to autophagy correlated transformation of the microtubule correlated protein 1A/1B light chain 3B (LC3B), that portray protein factors key for mitophagy. PINK1 represents a serine /threonine kinase, in addition to a labile protein. Nevertheless, its stabilization takes place on mitochondria which are depolarized, as well as its accrual have the capacity of enrolment in addition to activation of Parkin, an E3 ubiquitin ligase for facilitating the ubiquitination of the mitochondrial outer membrane proteins for stimulating mitophagy. Activation of Parkin by HBV further possessed the capacity of breaking down of mitofusin 2, which portrays a modulator of mitochondrial fusion along with start mitophagy.47 Mitophagy induction by HBx possesses the capacity of amelioration of mitochondria correlated apoptosis,47 as well as results in metabolic switch to glycolysis.52 The way detailed earlier MARCH has the capacity of being an antagonist for HBx induction of the mitophagy, which is an E3 ubiquitin ligase binding to HBx existent on the mitochondrial membranes for the induction of their breakdown.38 Intriguingly, despite HBx stimulated mitophagy the large (L) - HBsAg was further illustrated recently to be hampering sorafenib stimulated mitophagy through the WNT7B /CTNNB1 signaling pathway; thereby it might possess a part in chemotherapy resistance in case of HCC.53
HBV preS2 mutant along with mitochondrial metabolism
The HBV middle (M) HBsAg is not imperative for viral replication.54 HBV mutants possessing in frame deletions at the N-terminal of the preS2 coding sequence implicating M HBsAg ATG codon are usually identified from patients with the manifestation of the Chronic HBV at the time of natural infection. Such preS2 mutants do not possess the capacity of expressing M HBsAg; however express an L HBsAg that possesses an internal deletion possessing a T cells as well as B cell epitopes.55 In view of the elimination of T cells as well as B cell epitopes in the L HBsAg HBV preS2 mutants plausibly portray immune escape mutants in addition to are correlated with greater robust liver diseases inclusive of HCC.A recent study illustrated that preS2 mutants might result in endoplasmic reticulum (ER) stress, decrease the MMP, in addition to ATP generation resulting in calcium overload that might be correlated with escalated pathogenicity.54
Actions of HBV on the metabolism of the CD8+T cells
CD8+cytotoxic T lymphocytes (CTL) possess a key part in the elimination of HBV from the subjects having infection. Nevertheless, subjects having Chronic hepatitis B possess T cells which have been exhausted functionally with the impairment of the mitochondria.55,6 Actually in their recent study Tian et al.,57 observed quantities of programmed cell death (PD-1)-an immune checkpoint protein, in mice having continuous HBV replication in addition to their impairment of function was present.59 Fisicaro et al.,57 contrasted the transcriptomic profiles of the HBV- particular CD8+T cells that had been removed from subjects who had acute as well as chronic disease with that of the patients having HBV- infection which had resolved.57 Furthermore, utilization of influenza particular CD8+T cells which were identified from healthy subjects in the form of controls for virus was done by them for contrasting. Their observations were that exhausted HBV- particular CD8+T cells possessed remarkable mitochondrial changes in addition to improvement of antiviral activity of such CD8+T cells was feasible by the utilization of antioxidant which had the capacity of targeting mitochondria, pointing to the part of the ROS in case of exhaustion of the T cells.57 These outcomes were validated by Acerbi et al.,58 from the same group as Fisicaro et al.57 Thus there group evoked the probability of mitochondrial targeting with the idea of treatment of the subjects having chronic hepatitis B.
The escalated generation of the ROS by mitochondria having impairment might result in injury to the DNA. Montali et al.,60 recently revealed that exhausted HBV- particular CD8+T cells possessed escalated DNA injury in addition to impairment of healing.60 DNA injury was correlated with an escalated expression of the CD38 alias cyclic ADP ribose hydrolase, that portrays a main NAD utilizer , in addition to continuous activator of poly ADP ribose polymerase 1(PARP1), which results in escalated utilization of NAD along with its elimination. This elimination of NAD resulted in the dysfunctional poly ADP ribosylation (PARylation) which is significant DNA for the damage response (DDR) as well as aggravated cellular impairment of exhausted HBV- particular CD8+T cells (Figure 3A).
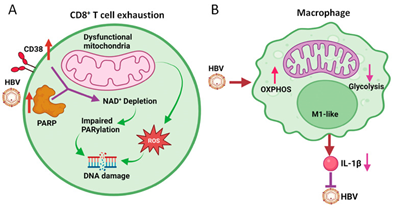
Figure 3 Courtesy ref no-3-Effects of HBV on mitochondrial metabolism of CD8+ T cells and macrophages.
(A) HBV induces the expression of CD38 and PARP1, resulting in the depletion of NAD+ and the subsequent inhibition of PARylation to cause DNA damage. ROS generated by dysfunctional mitochondria also cause DNA damage. This results in CD8+ T cell exhaustion.
(B) HBV induces OXPHOS and inhibits glycolysis in M1-like macrophages to attenuate the production of IL-1β, which inhibits HBV replication.
Further studies revealed that the restoration of the NAD quantities had the capacity of replenishing CD8+T cells working.61 Intriguingly, Schmidt et al.,62 illustrated that the hampering of acylCoA: cholesterol-acetyltransferase (ACAT) that is responsible for the catalysing as well as esterification of cholesterol along with the storage in lipid droplet ( LDs) in the cytoplasm, might escalate OXPHOS in addition to the generation of the ATP; thus rescue of the HBV- particular CD8+T cells ex vivo which are impaired.62 Thereby the manipulation of the mitochondrial metabolism opens potential new vistas for the treatment of chronic hepatitis B subjects. The manner HBV impacts the mitochondrial metabolism of the CD8+T cells continues to be elusive, since HBV does not result in infection of these cells. It is plausible that this might implicate the actions of other immune cells as well as/or cytokines. Requirement of greater studies are existent for clarity of this.
Actions of HBV on the metabolism of the macrophages
Macrophages represent phenotypically plastic cells having the capacity of going through M1 pro inflammatory polarization/ M2 anti-inflammatory polarization possessing diverse metabolic profiles.63 M1 macrophages having the properties of capacity of generation of the ROS in addition to pro-inflammatory cytokines inclusive of tumor necrosis factor alpha (TNF-α), Interleukin (IL-6), as well as IL-1β. They commonly reveal greater glycolysis actions along with lesser OXPHOS actions. Compared to M1 macrophages, M2 macrophages have the properties of expression of CD163 ( a member of the scavenger receptor super family) ,mannose receptor C type1 (MRC1) along with IL-10 (anti-inflammatory cytokine reveal lesser glycolysis actions along with greater OXPHOS actions.64–66 Nevertheless, recent studies have pointed that this is too simplistic classification in the form of M1/ M2 variation of macrophage phenotypes.64 In case of HBV infection macrophages possess a key part in the clearance of the virus in addition to its persistence. Utilization of transgenic mice aided this group of Li, Ou and colleagues59 to illustrate those mice which were geno-typically HBV negative as well as were born to hemizygous HBV transgenic dams possessed immune-tolerance to the HBV. Thereby HBV possessed the capacity of generation of continuous replication in these mice on introduction of the HBV genomic DNA into the liver of these mice by hydrodynamic injection.59 Compared to that HBV did not possess the capacity of generation of continuous replication in mice which were born to HBV negative dams. These outcomes pointed that maternal antigen impacted the immunity of the offspring in addition to reasoned out why mother-child transmission alias vertical transmission usually results in lifelong chronic hepatitis infection in children. On the other hand, the horizontal transmission amongst non-relatives usually results in self restricted infection.10 Further studies this group of Li, Ou and colleagues, pointed that mouse Kupffer cells (KC’s),alias resident macrophages in liver went through M1like polarization on exposure to the maternal HBV antigen, however went through M2 like polarization in case of non-exposure.19,68 These observations were in agreement with previous documentation, that pointed that chronic hepatitis B virus infection in a humanized mouse model which was grafted with the human hepatocytes in addition to, hepatopoietic stem cells were correlated with greater quantities of the infiltrating M2 like macrophages.69 They were further in agreement with the clinical findings that subjects having the quantities of the HBV DNA as well as greater quantities of the ALT possessed the greater CD16+ monocytes along with/or macrophages in the peripheral blood in addition to liver pointed to the state of the immune activation. CD16+ represents a of the M1 like macrophages.70
HBV further possesses the capacity of changing the metabolism of the macrophages as well as thereby change their working, resulting in impairment of the immune reactions along with the escalated predisposition infection.71,72 The interference with mitochondrial metabolism to the macrophages might result in reduction in the generation of the energy in addition to escalated OS.30 This might possess a variety of the negative actions in the working of the macrophages inclusive of dysfunctional macrophages, diminished generation of the cytokines as well as changed polarization. Performance of the RNA-seq for the evaluation of the actions of the HBV on the macrophages obtained from the THP1 cells, a monocytes cell line by group of Li, Ou and colleagues, it was observed that HBV stimulated the induction of the enzymes implicated in OXPHOS.67
Further assessment in addition to the estimation of the oxygen consumption rate (OCR) along with the extracellular acidification rate (ECAR) [which portray OXPHOS actions in addition to glycolysis actions respectively) validated that HBV possessed the capacity of induction of the OXPHOS as well as hampering of the glycolysis in case of THP1 macrophages (Figure 3B). Akin outcomes were obtained once KC’s identified from the naïve mice having hydrodynamic injection with the HBV genomic DNA. This KC’s revealed M1 phenotypes. These observations that HBV possessed the capacity of induction of the OXPHOS in case of M1like macrophages is quite astonishing in view of earlier studies pointed that M1like macrophages possessed escalated glycolysis actions in addition to lesser OXPHOS actions.64 Their observations suggested that metabolic reprogramming in macrophages is complicated as well as various external stimuli possessed the capacity of resulting in various metabolic results. In view of the treatment of the macrophages with utilization of dimethyl malonate (DMM), that hampers oxidation of succinate for the hampering of OXPHOS diminished the expression of IL-1β, such OXPHOS induction by HBV might decrease the generation of IL-1β by macrophages.67 These observations are of significance, in view of IL-1β by the binding of its receptor on the hepatocytes possessed the capacity of repressing HBV gene expression in addition to HBV replication (Figure 3B).67 Thereby utilization of OXPHOS induction in the macrophages is done by HBV for the amelioration of this anti-viral reaction. In view of HBV generating cell lines which got c cultured with macrophages utilizing Trans well possess the capacity of induction of the OXPHOS of macrophages through antigens liberated from the cells.
Mitochondria possess significant part in the generation of ATP, innate immune reactions along with apoptosis. More recently studies in the last decade have displayed that utilization of plethora of pathways is done by HBV for influencing the mitochondrial metabolism in the hepatocytes in addition to immune cells. HBV possesses the capacity of changing the physiology as well as metabolism of the mitochondria for the induction of the calcium signaling in addition to interfere with host innate immune reactions. It further has the capacity of facilitating fission along with mitophagy as well as result in repression of anti-viral actions of immune cells. These actions of the HBV on the mitochondria facilitate HBV replication, continuation in addition to pathogenesis. In fact metabolic pathways have been further unravelled to be targeted for the generation of the agents for the treatment of chronic hepatitis B infection. Future studies would further help in translation of this knowledge for its use in clinical scenario for final eradication of cccDNA completely. Restoration of HBV- particular T cell immunity has been recognized in the form of an attractive strategy for the functional cure of chronic Hepatitis B (CHB), which makes it imperative to form valid assays for invigorating as well as monitoring HBV- particular T cell reactions in patients with CHB. Fu et al.,73 recently evaluated hepatitis B virus (HBV) core- as well as envelope (env)- particular T cell reactions utilizing in vitro expanded peripheral blood mononuclear cells (PBMCs) from patients with CHB illustrating separate immunological phases, inclusive of immune tolerance (IT), immune activation (IA), inactive carrier (IC), along with the HBeAg-negative hepatitis (ENEG). Additionally, they assessed the actions of metabolic interventions, inclusive of mitochondria-targeted antioxidants (MTA-mito TEMPO, MitoQ), polyphenolic substances (resveratrol, oleuropein) in addition to ACAT hampering agent (iACAT) [avasimibe, K-604), on HBV- particular T-cell working capacity. They observed that HBV core- as well as env- particular T cell reactions were substantial in agreement along with of greater intensity in IC as well as ENEG in contrast to that in the IT along with the IA stages. HBV env-particular T cells possessed greater impairment however predisposed to react to metabolic interventions using MTA, iACAT, in addition to polyphenolic substances in contrast to HBV core- particular T-cells. The responsiveness of HBV env-particular T cells to metabolic interventions might be anticipated by the eosinophil (EO) count along with the coefficient of variation of red blood cell distribution width (RDW-CV). Thereby concluding that such observations might yield significant knowledge for metabolically HBV- particular T-cells for the treatment of CHB (Figure 4).73
Furthermore, recently Wang et al.,74 comprehensively elaborated the part of mitophagy and its implications in human diseases where they stressed on the working of the NLRP3 inflammasome with regards to the clearance of viruses. Earlier we had comprehensively reviewed part of NLRP3 inflammasome in reproduction, type 1 & 2 diabetes mellitus (DM), obesity.75 Different new studies have displayed that viruses possessed the capacity of particularly controlling the balance amongst mitophagy as well as the NLRP3 inflammasome for facilitating their replication. Parkin knockout mice reveal escalation of innate antiviral inflammation in addition to enhanced viral clearance via escalating mtROS-mediated NLRP3 inflammasome activation. Furthermore elimination of NLRP3 reverses the boosted antiviral reactions in Parkin knockout mice. Escalating mitophagy apparently is a promising approach for viruses for the prevention of the early clearance by NLRP3 inflammasomes. Influenza a virus protein PB1-F2 is translocated to mitochondria via translocases of OMM (TOM40) channels. It diminishes MMP for the induction of mitochondrial fragmentation, thereby ameliorating the RIG-I signal along with the disturbance of the NLRP3 inflammasome pathway. Acknowledged that RIG-I-like receptors are sensors which start along with control antiviral immunity, decreasing their signal results in dysfunctional clearance of influenza a virus (IAV). Nevertheless, as per certain documentation, receptor interacting protein kinase 2 (Ripk2) activates mitophagy by phosphorylation of ULK1. Knockout of Ripk2 in mice results in dysfunctional mitophagy, escalates NLRP3 activation, along with and enhances mortality upon influenza a virus infection. Noticeably, this is just a single presentation of pathogenicity to host cells subsequent to robust progression of influenza a viruses. Moreover, HIV long terminal repeat region (ssRNA40) possessed the capacity of hampering mitophagy from activating the NLRP3 inflammasome in human primary microglia, facilitating neurotoxicity in addition to neurodegeneration by escalating ROS generation. Injection of berberine into IAV-infected mice ameliorates inflammatory lesions in the lungs by activating mitophagy as well as hampering NLRP3 inflammasome activation. We need to further evaluate part of NLRP3 inflammasome in HBV and how mitophagy might aid in treatment of chronic hepatitis B infection. They further emphasized on the significance of how mitochondrial biogenesis is associated with mitophagy was highlighted in Figure 5.74
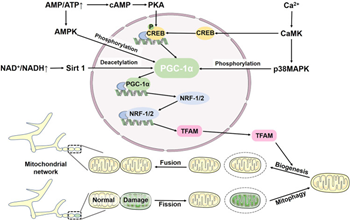
Figure 5 Courtesy ref no-74-Mitochondrial biogenesis and mitophagy jointly maintain mitochondrial homeostasis. Mitochondrial biogenesis is regulated by the PGC-1α-NRF-1/2-TFAM pathway. Elevated AMP activates AMPK, which directly phosphorylates PGC-1α, increasing expression of PGC-1α and TFAM. In addition, AMP can be converted to cAMP, which regulates PGC-1α through the cAMP-PKA-CREB pathway. Ca2+ stimulates CaMK to phosphorylate p38 MAPK. Additionally, CaMK can stimulate PGC-1α via CREB. In response to NAD+, Sirt1 deacetylates PGC-1α to activate PGC-1α.
None.
None.
The author declares that there are no conflicts of interest.

©2024 Kulvinder, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.