Journal of
eISSN: 2373-6453


Research Article Volume 3 Issue 3
1Department of Medical Laboratory Sciences, Imperial Post Graduate Medical Institute, Imperial College of Business Studies, Pakistan
2The Children's Hospital and Institute of Child Health, Pakistan 3Department of Zoology, The Women University, Pakistan
Correspondence: Muhammad Imran, Department of Medical Laboratory Sciences, Imperial Post Graduate Medical Institute, Imperial College of Business Studies, Lahore, Pakistan, Tel 00923320978431
Received: May 19, 2016 | Published: June 6, 2016
Citation: Anwar K, Imran M, Shahzad F, Noreen M, Atif M, et al. (2016) Prevalence of Hepatitis B and Hepatitis C Infection among Patients UndergoingDialysis. J Hum Virol Retrovirol 3(3): 00094. DOI: 10.15406/jhvrv.2016.03.00094
Hepatitis B and Hepatitis C are the major causes of morbidity and mortality in Pakistani population and their influence is considerable in patients suffering from kidney diseases and undergoing dialysis (Haemodialysis/ Peritoneal). This study considered the total number of dialysis a patient received and the number of dialysis in a week with the chances of Hepatitis C and Hepatitis B infection. This cross sectional study was conducted to determine the frequency of hepatitis B and hepatitis C in patients undergoing either haemodialysis or peritoneal dialysis. Serum sample was extracted from the blood of 60 patients. All the patients were suffering from either chronic kidney disease or acute renal failure. Screening was performed by strip method, which is based on Immuno-chromatographic technique for the detection of antibodies against HBsAg and HCV in patients on dialysis (haemodialysis/ peritoneal dialysis). The patients undergoing haemodialysis or peritoneal dialysis were randomly selected, out of which 43 were males and 17 were females. The association of HCV and HBV infection was calculated in relation to frequency per week of dialysis and total number of dialysis. There is significant correlation between HCV infection and total numbers of haemodialysis a patient has. Increase in the frequency of HCV infection was observed as the number of dialysis increased..
Keywords:Hepatitis B, Hepatitis C, Dialysis, Prevalence, ELISA, Hepatitis C infection, HCV
HCV, Hepatitis C Virus, HD, Haemodialysis, WHO, World Health Organization, HBV, Hepatitis B Virus, HBsAg, Hepatitis B Surface Antigen, RIA, Radioimmunoassay, EIA, Enzyme Immunoassays
Hepatitis C is caused by blood borne pathogen the Hepatitis C Virus (HCV). About 28% frequency of HCV caused by dialysis was reported in Pakistani population.1 HCV infection is becoming a major public health problem, with an estimated global prevalence of 3% occurring in about 180 million carriers. Approximately 4 million new cases are reported annually.1 There has been a strong association of haemodialysis (HD) and HCV infection. It seems an important contributing factor for spread of hepatitis. A similar correlation was observed between HBV or HCV marker positivity and the number of patients treated per haemodialysis unit.2
Diagnosis of hepatitis C virus infection is based on documented anti HCV antibodies. A small proportion of acute HCV infections (and chronic infections as well) are serum negative as determined by ELISA. This can occur in patients with impaired immunity, which cannot generate a detectable level of anti HCV antibodies or in whom antibody production is delayed. HCV-RNA detection by a sensible method when anti HCV antibodies tests are negative, suggests an acute hepatitis C virus infection, especially when it is followed by anti HCV serum conversion3 An estimated HCV prevalence of 3.9 million people was found in the United States with 2.7 million people found to have chronic infection with HCV (positive HCV RNA). Neither sex nor racial-ethnic group was found to be independently correlated with HCV infection.4
Long term dialysis is one of major factors leading to HCV. The natural history of HCV infection in dialysis patients remains incompletely understood. Controversy continues even in patients with intact kidney function. Prevalence of confirmed serologic status for anti-HCV antibody in blood donors ranges from less than 0.1% in Northern Europe to 0.5% in Western Europe and North America. Higher rates have been reported from Brazil, Eastern Europe, the Mediterranean area, and parts of Africa and Asia (1%-5%).5
In contrast to HBsAg, HCV positivity is high as 0.7% and 18.1% in haemodialysis versus peritoneal dialysis (PD) populations (7.9% ± 5.5% versus 3.0% ± 2.0%, p= 0.01). High rate of death is associated to it (Figure 1). HD related presence of anti-HCV antibody was dominant in Japan and was an independent predictor of mortality (relative risk 1.37, 95% CI 1.15-1.62, P = 0.003). In Australia and New Zealand, all-cause mortality was predicted by both the presence of anti-HCV at baseline (adjusted hazards ratio 1.29, 95% CI 1.05-1.58, P = 0.016) and the development of anti-HCV antibodies during the course of dialysis (adjusted hazards ratio 1.27, 95% CI 1.04-1.55, P = 0.017).6
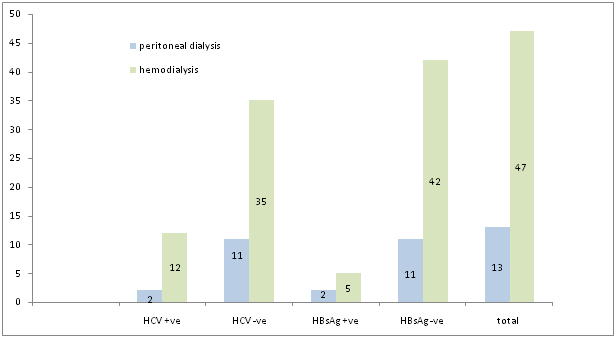
Figure 1 Frequency of HCV & HBsAg in patients undergoing haemodialysis & peritoneal dialysis (n = 60).
Hepatitis B is another serious public health problem. There are about 2 billion individuals infected worldwide and 350 million are living with chronic HBV infection. The World Health Organization (WHO) estimates that 500,000 to 1.2 million deaths each year are due to HBV related chronic liver disease and this infection is the tenth leading cause of death. Hepatitis B infection can occur in all age groups and its transmission is complicated.7 Haemodialysis patients are at high risk of acquiring parentally transmitted infections not only because of the large number of received blood transfusions and the invasive procedures that they undergo but also because of their immunosuppressed state. Several reports have been published about prevalence in haemodialysis patients ranging from 0% to 36%.8
Occult hepatitis B virus (HBV) infection can be defined as the presence of HBV DNA in the liver and/or blood in the absence of detectable serum hepatitis B surface antigen (HBsAg). There is a high prevalence of occult HBV infection in dialysis patients.9 HBV infection is associated with sufficient changes in the serum levels of hepatitis B antigens and antibodies that are responsible for different clinical states. The HBsAg is the serologic hallmark of HBV infection. It can be detected by radioimmunoassay (RIA) or enzyme immunoassays (EIA).10 The only generally approved treatment for chronic hepatitis B was alpha interferon, which is a natural antiviral agent but acts primarily by immunomodulation. Interferon treatment is associated with considerable but tolerable side effects in approximately 90% of the patients.11
HBV and HCV infections are associated with significant public health issues globally. They are important causes of morbidity and mortality in hemodialysis patients. Because the two hepatotropic viruses share same modes of transmission, co-infection with the two viruses is common, especially in areas with a high prevalence of HCV infection and among people at high-risk for parenteral infection.12
Chronic infections with HBV and HCV are associated with serious health risks due to hepatic cirrhosis and hepatocellular carcinoma. Patients undergoing HD therapy are at increased risk for acquiring these infections and have a higher prevalence of HBV and HCV than the general population. Global data indicate that the prevalence of HBV and HCV infection is high in populations of Africa and the Middle Eastern regions. HCV infection was estimated by WHO to affect 4.6% of the Eastern Mediterranean population and 5.3% of the population of Africa.13
This study is conducted to determine the frequency of hepatitis B and C in patients undergoing dialysis. This cross-sectional observational study was carried out in time period of five months in Children’s Hospital and Institute of Child Health Lahore, Pakistan. 60 patients without specification of the type of dialysis (PD/HD), age and gender were included in this study. Samples from the patients who had undergone multiple or >5times dialysis were included while those who were experiencing first session of dialysis were excluded. Consent of patient or patient’s guardian was taken and about 3 ml of patient’s blood sample was collected by a clean venipuncture. The blood was allowed to clot at room temperature. Blood sample was centrifuged at 2000 rpm for 2 min and serum was separated. Serum was processed with immunochromatography technique for detection of HBsAg and HCV antibodies and results were analyzed.
HEXAGON HBsAg 1-STEP - Omega Diagnostics kit (catalog number OD047) was used for the qualitative detection of HBsAg in human serum or plasma. The test is based on an immunochromatography. Test kit and samples were brought to room temperature prior to use. Test strip was holded only at the upper end which has an arrow pointing downwards. Container was immediately closed after removal of test. Test strip immersed with the arrow pointing downwards into a tube with 0.25 to 0.5 ml sample. The sample was not touching the cover foil of test. The test was not removed until the chromatographic process was complete. The results were read within 20-30 minutes after immersion into the sample at a well lit place.
Genomix HCV One Step test device (Catalog number: GNM 02-07-220) was used for the detection of HCV. The HCV one step test device is a qualitative, membrane based immunoassay for the detection of antibody to HCV in serum or plasma. Test device, specimen, buffer and controls were allowed to equilibrate at room temperature prior to testing. The test device was removed from the foil pouch and used very soon. The assay was performed within one hour. The test device placed on a clean and level surface. The specimen was transferred by a pipette or a dropper. 5 micro liter of serum or plasma was transferred to the specimen well of the test device, then 2 drops of buffer (80 micro liters) were added. The results were noted 10 minutes after appearance of colored line.
60 patients with kidney diseases undergoing dialysis as part of their management irrespective of their age and gender were included in this study. Out of 60 patients 43 (71.66%) were male and 17 (28.33%) were females. Among 60 patients 47 (78.3%) were on haemodialysis and 13 patients (21.6%) were on peritoneal dialysis. 28 (46.7%) patients were dialysed <50 times, 22 patients (36.7%) were dialysed 50-100 times, 6 patients (10%) were dialysed 100-200 times and 4 patients (6.7%) were dialysed >200 times (Table 1).
Frequency of dialysis |
Number of patients |
Percentage (%) |
<50 |
28 |
46.7 |
50-100 |
22 |
36.7 |
100-200 |
6 |
10.0 |
>200 |
4 |
6.7 |
Total |
60 |
100 |
Table 1 Total number of dialysis (n = 60)
Most of the patients had frequency “once a week”. 3 patients (5%) were with frequency of dialysis “once a month”. 23 patients (38.3%) were with frequency “twice a week” and 4 patients (6.7%) were with frequency “thrice a week”. chi square test was applied on this data to calculate the prevalence of HCV and HBV in dialyzed patients. p=0.34 for HCV in relation to frequency of dialysis in week and =0.54 for HBV.
Out of 47 haemodialyzed patients, 5 patients (10.6%) were HBsAg positive and 42 patients (89.36%) were HBsAg negative. Similarly, out of 47 haemodialyzed patients 12 patients (25.53%) were HCV positive and 35 patients (74.46%) were HCV negative (Figure 2). 13 patients undergoing peritoneal dialysis, 2 patients (15.38%) were HBsAg positive and 11 patients (84.61%) were HBsAg negative. Similarly, out of 13 patients with peritoneal dialysis 2 patients (15.38%) were HCV positive and 11 patients (84.61%) were HCV negative (Figure 2). Out of total 60 patients, 7 patients (11.66%) were HBsAg positive and 53 patients (88.33%) were HBsAg negative. Similarly out of total 60 patients, 14 patients (23.33%) were HCV positive and 46 patients (76.66%) were HCV negative.
Out of total 28 patients with dialysis <50 times, 6 patients (21.42%) were HCV positive and 22 patients (78.57%) were HCV negative. 22 patients with dialysis 50-100 times 5 patients (22.72%) were HCV positive and 17 patients (77.27%) were HCV negative. Out of total 6 patients who were 100-200 times dialyzed 1 patient (16.66%) was HCV positive and 5 patients (83.33%) were HCV negative. Similarly, out of 4 patients <200 times dialyzed 2 patients (50%) were HCV positive and 2 patients (50%) were HCV negative. In our study (p=0.4) for HBV in relation to number of dialysis and (p=0.61) for HCV (Table 2).
Number of dialysis |
Screening results of HCV |
Total |
Screening results of HBsAg |
Total |
||
Positive |
Negative |
Positive |
Negative |
|||
<50 |
6(21.42%) |
22(78.5%) |
28 |
2(7.14%) |
26(92.8%) |
28 |
50-100 |
5(22.72%) |
17(77.27%) |
22 |
4(18.81%) |
18(81.81%) |
22 |
100-200 |
1(16.66%) |
5(83.33%) |
6 |
0 |
6(100%) |
6 |
<200 |
2(50%) |
2(50%) |
4 |
1(25%) |
3(75%) |
4 |
Table 2 Frequency of HCV and HBsAg in relation to number of dialysis in enrolled subjects (n = 60)
Out of total 28 patients with dialysis <50 times, 2 patients (7.14%) were HBsAg positive and 26 patients (92.85%) were HBsAg negative. 22 patients with dialysis 50-100 times 4 patients (18.18%) were HBsAg positive and 18 patients (81.81%) were HBsAg negative. Out of 6 patients 100-200 times dialyzed patients no patient (0%) was HBsAg positive so 6 patients (100%) were HBsAg negative. Similarly, out of 4 patients <200 times dialysed patients, 1 patient (25%) was HBsAg positive and 3 patients (75%) were HBsAg negative (Table 2).
Out of 47 patients undergoing haemodialysis there are 12 HCV positive and 35 HCV negative. In patients with haemodialysis, 5 patients are HBsAg positive and 42 patients are HBsAg negative. Similarly, out of total 13 patients undergoing peritoneal dialysis, 2 are HCV positive and 11 are HCV negative. Out of total 60 patients with dialysis (peritoneal/ haemodialysis) 14 patients are HCV positive and 46 are HCV negative. Similarly out of 60 patients, 7 are HBsAg positive and 53 are HBsAg negative.
Patients suffering from chronic kidney disease, acute renal failure, chronic renal failure and other end stage renal diseases are those who have complete or insufficient kidney functions to remove waste products from blood. Therefore they need a continuous artificial mechanism to clean their blood and for removal of harmful nitrogenous wastes that can damage body in different ways. Patients with renal diseases undergo dialysis. Frequency of dialysis may be once a week, twice or thrice a week. It depends on patient’s requirement. Due to multiple practices of dialysis, these patients are more prone to HCV and HBsAg infection.2
The data was collected on random bases and there is high male to female ratio (2.5:1) as 43 are male and 17 are female. Seropositivity ratio is also high in males as (3.6:3) in HCV and (2:2.5) in HBsAg. 11 HCV positive patients were male and 3 were female. Similarly, 5 HBsAg positive patients were male and 2 were female. A study supports our results also showed high male to female ratio as HCV seropositive percentages over 4 year were ranged from (6.9-9.0%) in males and (5.3-8.5%) in females.14-20
Literature review from different articles showed some relation in HCV/ HBV infection and variables such as frequency of dialysis in week, total number of dialysis and type of dialysis (HD/ PD). Although these variables are contributing factors toward HCV /HBV infection, but our study shows there is no significant association between HCV/HBV infection and our variables. Prophylactic vaccine against HBV has already been developed which can decrease its incidence. Comparatively, low rate of HBsAg positivity is evidence for effectiveness of HBV vaccine. Although, HBV are very serious public health problem with an estimated 2 billion individuals infected worldwide and 350 million with chronic HBV infection.9 All patients included in our study were vaccinated for HBV still seropositivity for HBV shows there is association of viral transmission and dialysis but not much significant.
The screening method for detection of HCV and HBsAg was immunochromatography strips. This method gives qualitative result about presence or absence of HCV or HBV infection. Some advance techniques, such as PCR and ELISA are more accurate and give quantitative results or viral load. Due to lack of resources we used only screening methods for detection of HCV and HBsAg.
The study was supported by Department of Medical Laboratory Sciences, Imperial Post Graduate Medical Institute, Imperial College of Business Studies, Lahore, Pakistan, The Children’s Hospital and Institute of Child Health, Lahore, Pakistan and Department of Zoology, The Women University, Multan, Pakistan.
None.

©2016 Anwar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.