Journal of
eISSN: 2373-6453


Review Article Volume 1 Issue 3
1Center for Technological Development in Health (CDTS)/National Institute of Science and Technology in Neglected Disease Innovation (INCT-IDN)-FIOCRUZ, Brazil
2Biochemistry of Protein and Peptides Laboratory, Oswaldo Cruz Institute-FIOCRUZ, Rio de Janeiro, RJ, Brazil
Correspondence: Salvatore Giovanni De-Simone, Center for Technological Development in Health (CDTS), Leonidas Deane bilding, room 309, Av Brazil 4365, Rio de Janeiro, RJ, Brazil
Received: July 27, 2014 | Published: September 2, 2014
Citation: Napoleà -Pego P, Gomes LP, Provance-Jr DW, De-Simone SG (2014) Mayaro Virus Disease. J Hum Virol Retrovirol 1(3): 00018. DOI: 10.15406/jhvrv.2014.01.00018
Mayaro virus produces a nonspecific, sublethal disease in man with symptoms that are often confused with dengue. The symptoms of arthralgia, often associated with these viral infections, can cause an incapacitating disability. To date, outbreaks have been localized and sporadic within the Pan-Amazonia forest since its first isolation in 1954 (Trinidad and Tobago). The available literature is diverse, scarce and dispersed. Mayaro virus is an alphavirus, which is phylogenetically related to the Semliki forest antigenic complex. In the New World, Mayaro and the related UNA viruses are the only members of this complex that have been isolated. The genome of Mayaro consists of single-stranded RNA with a positive charge and a length of 12 kb that can be subdivided into genomic and subgenomic regions that encode nonstructural and structural proteins, respectively. Mayaro has shown great plasticity in hosts for vertebrate infections, but high specificity in invertebrates towards the family Culicidae (mosquitoes). Risk factors for infection are forested areas in northern South America and the rainy season. Two genotypes of MAYV have been identified, L (restricted to Belterra, Brazil) and D (widely distributed in the Pan-Amazonia). The enzootic cycle is similar to the jungle cycle of yellow fever, which involves Haemagogus mosquitoes and monkeys as reservoirs. Of concern is the potential spread of the virus by the involvement of other secondary vectors and hosts such as Aedes aegypti, Aedes albopictus and Aedes scapularis that have been shown experimentally to efficiently transmit the virus. Together with the observed high viremia levels of infected individuals, a significant risk exists for an emerging disease in urban, rural and peridomestic locations close to enzootic foci of Mayaro virus.
Keywords: Mayaro virus, Arbovirus, Emerging disease, Viral structure, Immune response
AV, Alphavirus; SINV, Sindbis Virus; CHIKV, Chikungunya Virus; DENV, Dengue Virus; ELISA, Enzyme Linked Immunosorbent Assay; EEEV, Eastern Equine Encephalitis Virus; HI, Hemagglutination Inhibition; HLA, Human Leukocyte Antigen; MAYV, Mayaro Virus; ns, nonstructural protein; OROV, Oropouche Virus; PCR, Polymerase Chain Reaction; RT-PCR, Real-Time Reverse Transcription-PCR; ROCV, Rocio Virus; SFV, Semliki Forest Virus; VEEV, Venezuelan Equine Encephalitis Virus; YFV, Yellow Fever Virus; WEEV, Western Equine Encephalitis Virus
Arboviruses are a heterogeneous group of naturally occurring viruses that can be transmitted between vertebrate hosts by hematophagous arthropod vectors. Since 1963, this classification has been recognized by the International Nomenclature Subcommittee Viral.1 Today, more than 500 viruses are listed in the International Catalogue of Arboviruses and Other Vertebrates Viruses with approximately 150 species of arboviruses that infect humans and domestic animals.2,3 The arboviruses have a wide global distribution and are present in almost all continents (Figure 1), although they occur most frequently in the tropics.3,4 In South America, numerous epidemics have been attributed to arboviruses. In particular, Brazil has a geography that favors the spread of such viruses due to forests that cover more than 1/3 of its territory, high density of species that serve as vectors and a favorable climate that create ideal conditions for the maintenance of the viral cycle.1,5
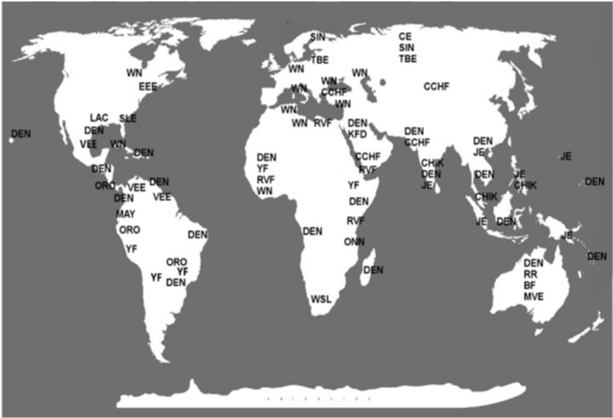
Figure 1 Time of intervention in HIV-1 life cycle for fusion inhibitors, HeLa/CD4-LTR-β-gal cells were infected with HIV-1IIIB cell-free virus before A) T-20 (100 μM), B) Bacitracin (3.5 mM) were added upon HIV-1 inoculation (time zero) or at various time points post-inoculation and β-gal activity was measured following 24 hr of incubation. Percentage values are relative to the positive control (no treatment). The data represent the means ± standard deviations from three separate experiments, each of which was carried out in duplicate.
The arbovirus persists in nature through life cycles that involve a variety of hosts including birds, rodents, primates, bats, reptiles, among others, as well as several arthropod vectors such as ticks, mites, black flies and horseflies, but primarily mosquitos.6 For most arboviruses, humans are accidental hosts due to their presence in forested areas. The only exception is the dengue virus that uses humans as its primary host to maintain cycles in urban and peri-urban areas.7 There are other factors that determine and influence the biological cycle of arboviruses beyond hosts, such as changes in the climate and ecological habitats that drive viruses to adjust to new reservoirs and vector species, which alter the prevalence of human infections and can increase the risk for the re-emergence of disease.4,8
In Brazil, over 210 species of arboviruses have been isolated with around 40 that cause sporadic, endemic and/or epidemic diseases in humans that are responsible for considerable morbidity and/or mortality. Most belong to the families Bunyaviridae (genus Orthobunyavirus), Flaviviridae (genus Flavivirus) and Togaviridae (genus Alphavirus). Some representatives of these families are the Oropuche Virus (OROV), Dengue Virus (DENV), Yellow Fever Virus (YFV), Rocio Virus (ROCV) and Mayaro Virus (MAYV), which together have been responsible for greater than 95% of human cases involving arboviruses in Brazil.7,8 The clinical features of arbovirus infections are in general similar and include fever, encephalitis, arthralgia, myalgia, rash or hemorrhagic fever.9,10 The absence of specific systemic symptoms makes it difficult to identify the etiologic agent in a clinical setting and many infections most likely pass without notice in the population, which together represent a serious public health problem.7,11
The family Togaviridae has only two genera, Rubivirus and Alphavirus, but they are responsible for a broad spectrum disease. Viruses in both genera are small, spherical and enveloped that measure between 60 and 70 nm in diameter.12,13 The genus Rubivirus includes about 30 different arboviruses, of which 11 are capable of causing disease in humans.14 Since the Rubivirus does not have a known invertebrate host, its transmission occurs primarily via aerosols, which interferes with its transfer between hosts. The genus Alphaviruses comprises a diverse group of 29 species that are nearly globally distributed and include three major categories: aquatic viruses, arthralgic viruses and encephalitic viruses.13 Except for the aquatic virus, all others have invertebrate hosts and are considered to have great relevance to public health. They include several infectious agents that are significant human and animal pathogens, some with the potential to be used as agents of bioterrorism.11,15
The Old World species include Alphavirus Sindbis (SINV), Semliki Forest Virus (SFV), Chikungunya Virus (CHIKV), O’nyong-nyong Virus and the Ross River Virus (RRV) that together are responsible for thousands of human cases worldwide. The New World Alphaviruses include important human pathogens for encephalitis such as the Western Equine Encephalitis Virus (WEEV), Eastern Equine Encephalitis Virus (EEEV) and Venezuelan Equine Encephalitis (VEEV). The symptoms presented by infections from these viruses are very similar to those of the Old World species and cause acute encephalitis in humans and domestic animals.7,16-18
In general, Alphaviruses are enveloped arboviruses possessing an icosahedral capsid enclosed in a lipid envelope derived from the plasma membrane of the host cell during budding and contains the viral proteins that organize into the capsid by forming trimers (Figure 2). Each trimer consists of three heterodimers formed between two viral glycoproteins.11,13,19 The genome consists of a single, positive strand of RNA and, unlike other viruses, has a polyadenylated 3’ end.20,21 There are approximately 11,700 nucleotides representing eight genes that encode the nonstructural proteins nsP1 to nsP4, which are involved in viral replication, the structural glycoproteins E1 and E2 of the envelope as well as the capsid and two small polypeptides E3 and 6K.22,23
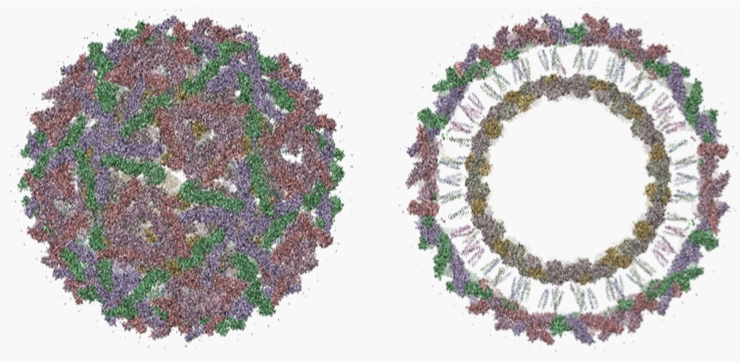
Figure 2 Time of intervention in HIV-1 life cycle for reverse transcriptase inhibitors, HeLa/CD4-LTR-β-gal cells were infected with HIV-1IIIB cell-free virus before A) UC781 (70 nM), B) AZT (20.0 μM) were added upon HIV-1 inoculation (time zero) or at various time points post-inoculation and β-gal activity was measured following 24 hr of incubation. Percentage values are relative to the positive control (no treatment). The data represent the means ± standard deviations from three separate experiments, each of which was carried out in duplicate.
The coat proteins of the alphavirus organize into spikes and have specializations that aid in maintaining the structure of the virus capsid through interactions with the viral RNA.24 During virus replication, they are inserted into the lipid bilayer of the host cell to form the viral envelope. The constituents of the viral envelope play an important role during virus entry into the host cell, which is thought to occur by receptor-mediated endocytosis followed by fusion of the viral envelope with the endosomal membrane25 or by direct penetration from the cell surface in the absence of membrane fusion.26 Although the overall infection cycle in mammalian and mosquito cells is similar, there seem to be some different requirements for cellular factors from the host cell.
The virus contains 80 such spikes, each consisting of two or three subunits from two of the type I transmembrane glycoproteins, E1 and E2 (± 50 kDa). Their association is through non-covalent interactions that create a stable complex. In some species of alphaviruses, another protein called peripheral E3 (10 kDa) is also present. The glycoproteins E2 and E3 are synthesized as a precursor, called p62 in SFV and PE2 in SINV. The cleavage of the precursor is performed by host cell proteases.27 In Brazil, the alphaviruses of highest priority are the Venezuelan Equine Encephalitis, Eastern Equine Encephalitis and Western equine encephalitis that cause equine encephalitis along with MAYV that is responsible for causing the Mayaro fever.8 Epidemiological studies on alphavirus infections are restricted due to insufficient surveillance and laboratory diagnostic analyses in most endemic countries, which result in an underestimation of the numbers of cases.28
Mayaro virus (Togaviridae, genus: Alphavirus) circulates as a zoonotic virus that is transmitted by arthropod vectors. It was first isolated in Trinidad (1954) from blood samples of five rural workers that had presented with fever.29 The virus is found exclusively in the Americas, particularly in countries with extensive tropical forests, such as Trinidad and Tobago, Peru, Guyana, Cooperative Republic of Guyana, the Republic of Suriname, Colombia, Venezuela, Panama, Costa Rica, Mexico, Bolivia and Brazil (Figure 3).17,30,31
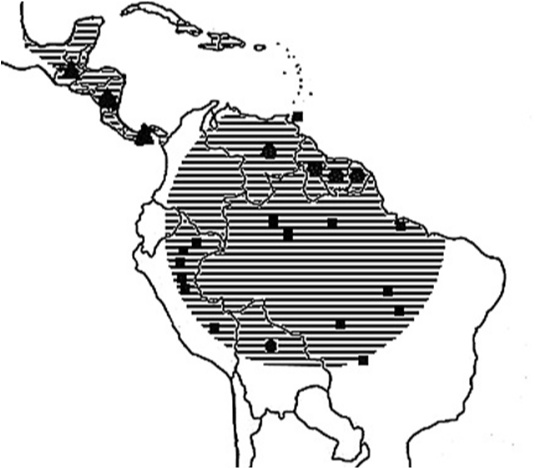
Figure 3 Time of intervention in HIV-1 life cycle for protease inhibitors, HeLa/CD4-LTR-β-gal cells were infected with HIV-1IIIB cell-free virus before A) Amprenavir (0.1 mM), B) Indinavir (0.25 μM) were added upon HIV-1 inoculation (time zero) or at various time points post-inoculation and β-gal activity was measured following 24 hr of incubation. Percentage values are relative to the positive control (no treatment). The data represent the means ± standard deviations from three separate experiments, each of which was carried out in duplicate.
In Brazil, the MAYV was initially isolated in 1955,32 and has since been responsible for several outbreaks in the Amazon region, where it can be considered endemic.16,17 From 1955 to 2011, there were several cases of the disease reported from four outbreaks detected in the state of Pará. The first, came from a community of workers at a quarry. In 1978, 800 workers were infected during an outbreak. An analysis of the outbreak included the detection of antibodies against MAYV in birds and monkeys, which suggested the probable wild reservoir were monkeys. In 1981, a third outbreak occurred in Conceição do Araguaia, Pará.33
The first outbreak outside the State of Pará was recorded in 1987 in Goiás Itarumã. In 1991, two additional outbreaks were reported, one in the town of Fish Tocantins and the other in the city of Benevides, both in the state of Pará. In 2000, three infections were diagnosed in São Paulo in individuals who had visited the Camapuã region in Mato Grosso do Sul.34,35 Between 2007-2008, 600 blood samples were analyzed from residents of Manaus that presented with high fevers. In 33 cases, the presence of the virus was detected.36 A suggested factor that influenced these outbreaks was the changes in the ecosystem associated with anthropogenic changes in forested areas.21 However, the expansion in the geographic range of alphaviruses and increased worldwide travel are also of emerging public health concern.37,38 Of note are four cases described in Europe of patients with a travel history to South America.31,39-42 The importation of alphaviruses into non-endemic regions can delay proper treatment since doctors are not familiar with the disease and reliable diagnostic methods are lacking at large.
MAYV infections are associated with a highly debilitating clinical condition characterized by fever, headache, diarrhea, vomiting, myalgia, arthralgia, rash and, in some cases, an uncharacteristic light bleeding.17,30,31 Even with a low mortality rate, the Mayaro fever is considered one of the most important arboviruses, being responsible for the fourth greatest number of reported cases after dengue, yellow fever and Oropouche. In 2011, it was included on the list of diseases requiring notification to the Ministry of Health of Brazil. Its signs and symptoms are quite similar to yellow fever and dengue (emerging and re-emerging public health problems), which leads to many cases of infection with Mayaro being misdiagnosed as those illnesses.17,35
The maintenance cycle of MAYV resembles that of other arboviruses with a sylvatic cycle maintained in bloodsucking mosquitoes that serve as the vector to the wild hosts consisting of birds and non-human primates (Figure 4). Man is considered an accidental host from invading the habitat of sylvatic reservoirs.17 From seroepidemiological studies, the prevalence of MAYV in several mammalian species (anteaters, sloths, agoutis, marsupials, rodents) shows that vectors have a high capacity to disperse the virus to a wide range of hosts, which warns of the risks for new outbreaks. It is worth noting the possible involvement of synathropic animals in periurban environments.43,44
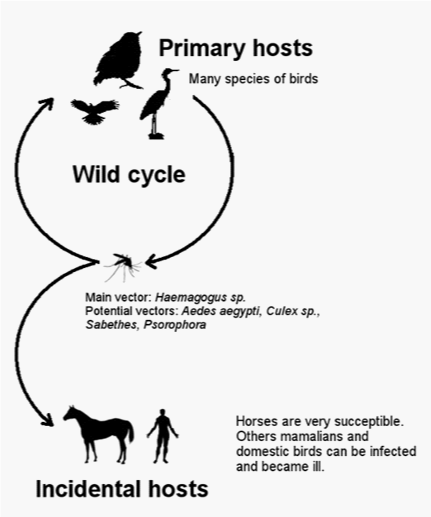
Figure 4 Time of intervention in HIV-1 life cycle for integrase inhibitors, HeLa/CD4-LTR-β-gal cells were infected with HIV-1IIIB cell-free virus before 118-D-24 (100 μM) was added upon HIV-1 inoculation (time zero) or at various time points post-inoculation and β-gal activity was measured following 24 hr of incubation. Percentage values are relative to the positive control (no treatment). The data represent the means ± standard deviations from three separate experiments, each of which was carried out in duplicate.
Its main vector species is the mosquito Haemagogus janthinomys. However, other genera of mosquitoes are also recognized as vectors, such as Culex and Psophors sabethes. Some studies have suggested Aedes aegypti as a potential vector, which should be considered a major public health problem since this mosquito is well adapted to the urban environment. Also, there is the possibility for the association of Mayaro fever with dengue through the use of the same insect as a host.17,28,35,45
There appears to be two different genotypes in circulation.45 The first, designated D, was isolated in Trinidad and Tobago, Peru, Guyana, Suriname, Bolivia and Brazil while the second, designated L, has only been observed in Brazil and shows a difference of 15-19% compared to genotype D. Since there was a low genetic diversity seen among the isolates within each genotype, it is believed that both are maintained by independent enzootic cycles. A comparison of the genetic relationships among the Marayo virus strains sequenced is shown in Figure 5.
At the molecular level, the MAYV is consistent to other alphaviruses and consists of an enveloped viral particle that is 69 ± 2.3 nanometers in diameter with an icosahedra nucleocapsid formed by 240 units of capsid protein.46 It has an RNA genome with 11,429 nucleotides (except poly-A tail). The first ORF (Open reading frame) encodes a polyprotein of 2436 residues, called P1234, that first undergoes cleavage to form the nonstructural protein 2 (nsP2).46-48 The second region of the ORF is translated from a subgenomic 26S RNA region and encodes a 1252 amino acid polyprotein. After undergoing cleavage by enzymes and furinas signalase present in the host, gives rise to proteins E1, E2, E3 and the 6k protein C (capsid) (Figure 6).25,46,47
The synthesis P1234 appears to activate the cleavage of nonstructural protein nsP4, allowing the formation of the complex P123/nsP4, which is capable of synthesizing RNA in the negative polarity and is complementary to the genomic RNA. The nonstructural proteins and host factors, such as enzymes, assist in the process of viral replication. The P123 polyprotein is then cleaved between the first and second junction to form the complex P1/P23/nsP4. Finally, separation occurs between proteins nsP2 and nsP3 that leads to the replication complex being formed by the nonstructural proteins, which are now completely processed.25,45
An initial analysis of the nucleotide sequence encoding the protein nsP1 and the noncoding region near the 3’ end of the 42S genomic RNA indicated that this complex belongs to MAYV Semliki Forest Virus (SFV), which includes viruses of the old world and new world.45,48
Overall, the MAYV virus is comprised of envelope glycoproteins and a lipid bilayer derived from the host cell membrane. This lipid-packed envelop is critical for the biological activity and stability of alphavirus particles.49 The process of forming virus particles involves each of its proteins that function at specific times and cellular locations, which have been extrapolated from observations of other viruses of the same family. The process begins with the translation of the 1242 amino acid structural polyprotein, p130. The amino acids 103-258 (peptidase S3) display serine protease activity that presents excellent physicochemical characteristics for cleaving linkages between tryptophan and serine. During ribosomal translation, this segment induces autocleavage that generates the C protein (capsid; aa 1-258), the precursor p62 (aa 259-746), 6K (aa 747-806) and the envelope glycoproteins E3 (aa 259-324), E2 (aa 325-746) and E1 (aa 807-1242). A small peptide of 15 amino acids (aa 259-273) resulting from multiple proteolytic events remains bound to ribosomes. The C protein and the two envelope proteins, E1 and E2, are type 1 transmembrane proteins and the 6K protein is a polytopic membrane protein. After cleavage, the peptide fragments remain transiently associated with ribosomes by binding to the viral RNA, which rapidly gathers particles into an icosahedral core. The resulting nucleocapsid eventually associates with the cytoplasmic domain of E2 in the cell membrane, leading to mature virions and release.
The p62 precursor is processed by a furin located in the host cell membrane just before the binding of virion for budding and gives rise to the heterodimer E2 - E1. The heterodimer p62 - E1 is stable, whereas E2 - E1 is unstable and dissociates in an acidic pH. p62 is processed in the last step, presumably to avoid an activation of the fusion of E1 before its final export to the cell surface. The C-terminal E2 segment contains a transient transmembrane region that can be interrupted by palmitoylation, resulting in a reorientation of the C-terminal tail from the lumen to the cytoplasmic side. The release of the C-terminal of E2 is involved in budding by interacting with capsid proteins and occurs late during the export of proteins, which prevents the premature assembly of particles in the endoplasmic reticulum membrane.
The 6K segment is a constituent of the cell membrane glycoprotein involved in the processing of the virus, cell permeabilization and budding of viral particles.25 It also disrupts the calcium homeostasis of the cell, probably at the level of the endoplasmic reticulum, leading to an elevated cytoplasmic calcium level. Due to its lipophilic properties, it is believed that the 6K protein may influence the selection of lipids which interact with transmembrane domains of the glycoproteins, which in turn affects the deformability of the lipid bilayer required for the extreme bending that occurs as a virus buds from the host cell. It is present at low quantities within mature viral particles in comparison to viral glycoprotein’s (~3%). Its location is not well defined, although it is sometimes found in association with proteins E1 and E2.
A heterodimeric of the two glycoproteins, E1 and E2, form trimers that are anchored in the membrane to form about 80 spikes on the surface of the viral envelope. The E2 protein is involved with cell recognition and viral adsorption. After attachment of the virus to the target cell and endocytosis, acidification of the endosome can induce dissociation of E1/E2 heterodimer. Protein E1 is a class II viral fusion protein responsible for fusion of the viral envelope with the endosomal membrane of the host cell.22 Its activity is inhibited when E1 is connected to E2, as observed in the spikes of the mature virion. The release of E1 homotrimers contributes and promotes the release of the viral nucleocapsid into the cytoplasm by its fusion of the viral and endosome membranes. Efficient fusion requires the presence of sphingolipid and cholesterol in the target membrane. It also interacts with the protein C on the inner face of the viral envelope.22,50,51
Although the function of the protein E3 is not well known,50 studies show that it has great affinity for ribosomes, which suggests its involvement in the translation of the viral genome in the cytoplasm. Also, in analyses with other alphavirus, it was observed that this protein plays a central role in the formation of viral structure through stabilizing the fusion proteins E1 and E2.45,52
While demonstrating a variable size from 258 to 275 amino acids, protein C has two separate areas of basic amino acids. One region, rich in lysine and arginine, most likely binds electrostatically to RNA. The other is a highly conserved domain has and has subregions that interact with the cytoplasmic domains. Protein C is responsible for capsid assembly and possesses a serine protease activity, which results in its autocatalytic cleavage and it can also cleave structural proteins.24,51,53
There are four different proteins in the family of nonstructural proteins and the individual function of each has been studied. Protein nsP1 has two types of enzymatic activities. The first is a 7-guanine methyltransferase and the second is guaniltranferase, both of which are necessary for the methylation of the ends of the genome and subgenomic RNA during transcription. It also appears to be important for the initiation and continuation of the synthesis of complementary RNA. The nsP1 is unique among the nonstructural proteins as a membrane associated protein. This association is due to the palmitylation of a cysteine that allows direct binding to hydrophobic residues. Another possible function of nsP1 is modulation of nsP2 proteinase activity.46,50
The nsP2 protein has several enzymatic properties. Its N-terminal has a domain with RNA helicase activity that functions during replication and transcription.25 The nsP2 also contains a domain with RNA triphosphatase activity and the C-terminal contains cysteine protease activity. The main function of nsP2 is to cleave at specific sites in the nonstructural polyprotein, which is produced early in the viral replication.53 Little is known about the protein nsP3, but it is believed to have an essential function for the synthesis of 26S subgenomic mRNA. Studies have shown that protein nsP3 may also have an important role in the transport of viral components.52
The protein nsP4 is an RNA viral polymerase that is highly regulated during viral infection, although some alphavirus do not appear to encode for nsP4.50 It is present in small quantities primarily because it has numerous tyrosines in its N-terminal region that confer instability and contribute to its rapid degradation.
Like most arboviruses, the MAYV is able to infect and replicate in cells from both vertebrates and invertebrates.27 The mechanisms involved in viral replication are not well known, and many studies have tried to reveal the cellular responses involved with infections by MAYV.53 Studies have demonstrated that the morphogenesis replication occurs in the cytoplasm.50 First, internalization of MAYV proceeds through vesicles destined for endosomes where acidification alters the viral envelope, leading to its fusion with the endosomal membrane (Figure 7). This event is followed by the release of the nucleocapsid into the cytoplasm where ribosomes bind to the positive sense RNA genome and begin translating the encoded polyprotein.27 The nonstructural proteins are the first to be produced and some host proteins assist in the replication complex. The genome is then transcribed into a negative sense RNA whose synthesis terminates four hours after an infection. This negative sense is later used to produce a genomic 49S RNA as well as a 26S subgenomic RNA that serves as the template for the transcription of the viral structural proteins.50
During translation of the C protein, a proteolytic cleavage site is exposed that after proteolysis releases a signal peptide for its translocation into the endoplasmic reticulum. The envelope glycoproteins are synthesized, glycosylated in the Golgi complex and transferred to the plasma membrane. Subsequently, the capsid proteins undergo self-assembly with the 49S genomic RNA and are associated with the regions containing the membrane envelope proteins. The maturation of the particles and budding occur mainly from vesicles with subsequent exocytosis of mature virions.53-56
Within invertebrate cells, infections can lead to the generation of inclusion bodies containing immature virions. The inclusion bodies have not been observed with budding virus. It is believed that the release of virions occurs by exocytosis, due to the large aggregates of viral particles present in the extracellular medium. Exocytosis could be an important mechanism for maintaining a state of MAYV persistent infection of invertebrate cells.45
MAYV infection is associated with a highly debilitating clinical presentation most often characterized by fever, arthralgia and rash.16,57,58 The earliest symptoms of an abrupt fever and arthralgia last an average of 3 to 5 days in the majority of cases.59 Arthralgia is a defining symptom with more intense pain in the small joints of the extremities.9 Other symptoms include headache, chills, myalgia, epigastric pain, nausea, back pain, diffuse pain, photophobia and retro orbital pain. Rash of the maculo-papular type are often observed. In some cases, the rash occurs widely, but in most patients, it is more intensely observed on the chest, back, arms and legs. The rash usually appears on the fifth day of disease and persists for three days.51,60,61
In most individuals, the fever is self-limiting and most symptoms disappear between 3 and 10 days progressing to resolution of the infection without relapse. However, the arthralgia symptom can persist over many months causing a disabling polyarthritis that, in some cases, can be associated with additional incidences of fever.16,36,61 There are reports of chronic and recurrent arthralgia for up to 6 months after a MAYV infection. Recently, other symptoms were described in Mexico for patients diagnosed with MAYV that showed bleeding, thrombocytopenia and jaundice. In addition, one died of encephalitis symptoms after three days.62,63
Currently, no studies related to the initiation of MAYV infection have been undertaken. However, the events are expected to share similarities to other alphaviruses. After subcutaneous delivery by a mosquito bite, alphaviruses appear to be disseminated in the host through lymph nodes and the microvasculature. Leukopenia in the acute phase of the disease is a very common hematologic alteration from an alphavirus infection, suggesting a primary replication of the virus in the leukocytes.17,64,65 Liver and spleen are also considered primary sites of viral replication,66 which contribute to its dissemination to bone, muscle, and articular tissues that are strongly associated with local inflammatory processes.67-69 Host age, the status of the immune system, virus strain virulence and viral persistence are all key determinants for the pathogenesis of alphavirus infection in animals.18,67 The severity of disease and persistence of symptoms are associated to the extent of virus replication and the presence of inflammatory mediators in the plasma for patients or in specific tissues for animal models.68
Interestingly, some cytokines secreted during an alphavirus infection are the same as those associated with the progression of rheumatoid arthritis (RA), although inflammation in RA is clearly associated to an autoimmune process, which has not been consistently demonstrated for alphavirus-induced arthritis.70,71 Despite particular differences, an expression analysis of inflammatory genes in a mouse model of CHIKV infection demonstrated similarities between the induced genes in that model to those induced in both RA and collagen-induced arthritis models.71 Also observed in alphavirus-induced arthritis were specific polymorphisms in the human leukocyte antigen (HLA) and autoimmunity development, two conditions previously associated to patients’ with a predisposition to rheumatic diseases and RA. In one example, the RA associated alleles HLA-DRB1∗01 and HLA-DRB1∗04 were identified in CHIKV chronic patients that were later diagnosed for RA.72 Some were positive for autoantibodies against the rheumatoid factor (RF), cyclic citrullinated peptide (CCP) and nuclear antigens suggesting a potential for CHIKV infections to initiate RA.72 Further evidence for a connection between alphavirus infections and RA come from SINV infections that also appear to associated with alleles of HLA involved in rheumatic diseases, in particular HLADRB1 ∗01.73,74 SINV-infected patients also showed elevated titers of autoantibodies, including anti-nuclear and mitochondrial antibodies, with significant increase in RF three years post infection.74 Moreover, HLA-DR7 has been shown to be increased in patients with polyarthritis following RRV infection.75 Taken together, these observations suggest that RA and alphavirus-induced arthritis share a set of common characteristics that could be useful in the development of new model systems for developing therapeutic approaches against arthritis.
Cellular and humoral immune responses are important in the control of primary infections with alphavirus.13 In the cellular immune response, the active participation of cytotoxic T lymphocytes are important for the lysis of infected cells and to produce ɣ-interferon for activation of macrophages for the production of other cytokines. These responses can influence viral replication and, consequently, the severity of the disease.53
Studies have demonstrated the existence of antigenic sites in common among alphaviruses. Some authors have reported that, in general, there are more antibodies directed against the structural protein E2 than to E1 protein, possibly because the E1 protein is more conserved.48 In addition, neutralizing epitopes have been described as well as epitopes that interact with specific antibodies.47 For Sindbis virus, a neutralizing epitope was identified in a linear stretch of E2 (aa 170-220) that is unique for alphaviruses. However, many epitopes are dependent of structural conformation of the protein to be reactive to antibodies, as in the example of the E1 protein. For the E2 protein, several studies show a need exists for denaturing or fragmentation of peptides for some their interaction with host antibodies.54
The appearance and persistence of antibody in hosts can vary considerably between patients. Furthermore, an infection with MAYV is capable of inducing antibodies IgM, which are usually transient and indicative of recent infection, but may persist for at least 90 days after the initiation of symptoms. In secondary infections, IgM production can occur at low levels. However, IgG, which persists throughout the life of the host, can be an excellent marker of the reoccurrence of an infection when found in high levels.36,55,56
Infections by MAYV are increasingly being considered a serious public health problem in rural areas and jungles of South America and can be easily confused with other arboviruses. There is a risk of MAYV becoming circulated within urban areas due to several factors: the expansion of ecotourism, increased occupancy and the possibility of Aedes aegypti become a vector MAYV. Moreover, the MAYV has been little studied,6 and although the proteins E1 and E2 have been identified as the most immunogenic in alphaviruses,25 little is known about the structural composition and antigenicity of other viral proteins. The applications of various proteomic tools including the microarray of epitopes can clarify the identification of antigenic determinants, their molecular structures and their interactions with the humoral immune system to improve the knowledge on the immunogenicity of each viral protein.
The signs and symptoms caused by MAYV are similar to symptoms of other arboviruses; especially chikungunya, yellow fever and dengue. This leads to many cases of Mayaro infection being misdiagnosed.6,8 Clinical diagnosis of MAYV infection is difficult because of the nonspecific nature of the prodromal illnesses and the presence of other viruses, such as DENV, ORO and CHIKV, which commonly produce identical diseases. Techniques based on the identification of the virus are difficult when samples are collected after the onset of symptoms since MAYV presents a short period of viremia of only 2 to 3 days. The most reliable method for the identification of arboviruses has, in general, been the isolation of the infectious agent.35 In the case of MAYV, laboratory diagnosis involves virus isolation from the in vivo inoculation into the brains of neonate mice or in vitro using cultured vertebrate cells (VERO, BHK-21) or invertebrate cells (Aedes albopictus clone C6/36).76
The most definitive test for MAYV infection and alphaviruses in general, is through virus isolation. In practice, this is not practical because it is time-consuming, expensive and requires subsequent diagnostic steps to identify the pathogen. Several serological techniques have been described for the diagnosis of Mayaro fever among them are the hemagglutination inhibition (HI) test, plaque reduction neutralization tests and complement fixation. The HI test is widely used as a routine diagnostic test due to its low cost and ease to perform, but has the disadvantage of requiring goose erythrocytes and requiring a second blood sample collected 15 days after the first collection to confirm a diagnosis. Complement fixation, which can identify antibodies that recognize MAYV, is more specific, but in comparison to the HI test it is less sensitive, more difficult to perform and is more costly. Enzyme linked immunosorbent assay (ELISA) is proving to be extremely useful in diagnosing of the virus due to its sensitivity and lower cost. An example is the IgM-ELISA antibody capture test (MAC-ELISA). Immunoglobulin M antibody responses, which are the most rapid and subtype specific, usually develop 4 to 5 days after infection and allows the recognition of active or recent diagnosis of infection from only a single serum sample collected in the acute phase of the disease.16,55 However, all of these diagnostic methods still have methodological and/or costs limitations and are not Mayaro specific tests.
In the last several decades, molecular biology techniques have been evaluated for diagnosis of several arboviruses. Among them, the polymerase chain reaction (PCR),77 and the multiplex nested PCR14 to amplify viral nucleic that are present in the blood of patients as early as two days post infection and normally are present up to 6 days. Most PCR-based methods, including a nucleic acid sequence-based amplification assay and real-time reverse transcription-PCR (RT-PCR), provide rapid and more sensitive means to detect alphaviruses, including MAYV.78,79 Recently, a specific RT-PCR amplification technique that provides for the fast, sensitive and genus-specific detection of all human pathogenic alphaviruses was tested.76 Two degenerate primers were designed to amplify a conserved region of the nsP1 gene of all alphaviruses, but sequencing or restriction enzyme digestion of the amplicon DNA was needed to identify the alphavirus amplified, limiting effectiveness. A nested PCR can be used for further confirmation, but this introduces greater potential for contamination and false positives. Unfortunately, no simple, rapid, specific and cost-effective diagnostic methods are currently available for detecting and distinguishing alphaviruses of different species, subtypes, and genotypes. Since minor genetic differences among strains can have profound epidemiological implications, the development of more specific diagnostic assays is critical.
Few documented cases of MAYV infections in man exist, yet it is an emerging risk due to the potential transfer of the virus from its normal geographical distribution within tropical rainforests to urbanized areas and the prospects of new, effective vectors and hosts. Arboviruses, as a whole, have a well-documented history of emergence through several mechanisms, often mediated through human transportation, and enhanced amplification in peridomestic commensal and domesticated animals leading to spillover to humans. Other contributing factors are urban expansion and global warming, which can increase the distribution of vectors and enhance the transmission potential within temperate climate zones by elongating transmission seasons, increasing host-vector contact by shortening vector gonotrophic cycles, and shortening extrinsic incubation times. The greatest risk for human disease is the ability of MAYV to adopt to new anthropophilic vectors, Ae. aegypti and Ae. albopictus, which could lead to anthroponotic urban or peridomestic transmission cycles involving highly efficient transfer between hosts. Without an effective vaccines, interventions would require improved control of urban vectors and surveillance, which would be complicated by similarities in the symptoms associated with MAYV infections and related viruses, such as dengue, along with the absence of rapid and reliable diagnostics. There is reason to believe that additional viruses such as VEEV and ZIKAV have the potential for urbanization, which could have devastating public health consequences, especially in the Western Hemisphere and in the case of Brazil from north to central and southeast areas of the country, where there is no herd immunity. Further research is needed to overcome the limited knowledge about the biology and immunology of MAYV for the development of a vaccine and to minimize the confusion in clinical setting for alphavirus diseases.
None.
None.

©2014 Napoleà -Pego, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.