Journal of
eISSN: 2373-6453


Review Article Volume 10 Issue 2
1Postgraduate Department of Zoology, Darrang College (Affiliated to Gauhati University), Tezpur, Assam, India
2Principal, Darrang College, Tezpur, Assam, India
3Department of Chemistry, Darrang College, Tezpur, Assam, India
Correspondence: Chittaranjan Baruah, Postgraduate Department of Zoology, Darrang College, Tezpur-784001, Assam, India, Tel +91 9954294080
Received: August 10, 2023 | Published: August 25, 2023
Citation: Baruah C, Saikia PM, Hazarika P, et al. Curcumin nanosystems as prospective antiviral alternatives: their stability in an aqueous Chitosan-Tergitol-15-S-7 system. J Hum Virol Retrovirol. 2023;10(2):50-54. DOI: 10.15406/jhvrv.2023.10.00266
Curcumin, the primary curcuminoid component of turmeric (Curcuma longa L), has been shown to have powerful antibacterial properties, inhibiting the growth of a wide range of infections. The research presented here focuses on current Curcumin nanosystems investigations to aid in the progress of curcumin and its derivatives as comprehensive antiviral therapies. The breakdown rates of curcumin were determined using spectrophotometry, which allowed the compound's stability to be determined using chitosan and Tergitol-15-S-7. Tergentole 5 was also utilised as a surfactant. Hydrophobic contacts, hydrogen bond formation, and electrostatic interactions are examples of exothermic interactions between curcumin and chitosan. Tergitol-15-S-7 impacts the interaction between curcumin and chitosan in large doses, according to an examination of absorption and fluorescence patterns at a physiological pH (7.4). The apparent binding constants and distribution of curcumin within the interior of chitosan have been demonstrated using the fluorescence quenching method. Fluorescence quenching techniques revealed that curcumin distribution in colloidal chitosan solution is not uniform. The hydrophobic interior of chitosan is mostly constrained to its cationic centres, which contain curcumin. Nano curcumin supplementation decreased inflammation, respiratory function, clinical symptoms, and sequelae in people with COVID 19 and other viral infections.
Keywords: curcumin, anti-viral, stabilization, chitosan, Tergotol-15-S-7, phytochemical, surfactant
Curcumin is one of the most extensively researched and promising chemicals created from dietary natural ingredients. Around 3000 preclinical investigations have been conducted on curcumin, and they have provided evidence of its potential benefits and safety (tolerated up to 12 g/day).1 Its biological properties include being anti-inflammatory, anticancer, antioxidant, depressive, and antiviral, according to scientific articles.1 Nano-curcumin supplementation improved inflammation, respiratory function, clinical symptoms, and outcomes in patients with COVID-19 viral infection.2
The primary phytochemicals of the turmeric-flavored Curcuma longa L. (Zingiberaceae family) rhizome are curcumin or diferuloylmethane, with the chemical formula 1,7-bis (4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione. Due to their extensive traditional usage and minimal side effects, Curcuma longa L. (Zingiberaceae family) and the polyphenolic component curcumin have been the topic of numerous antimicrobial investigations.3 Curcumin and C. longa rhizome extract have been shown to have antimicrobial properties against a variety of bacteria, viruses, fungi, and parasites. Many studies have been conducted to improve curcumin's antibacterial action, including the development of various chemical compounds to increase curcumin's water solubility and cell uptake. This study intends to summarise antimicrobial investigations on curcumin and its stabilization to apply it as a natural antiviral agent in upcoming studies.3
Curcumin is sensitive to photodegradation and unstable at different pH levels. Drug compounds with poor biopharmaceutical profiles may have their therapeutic potential increased with the aid of nanotechnology. Curcumin is photodegradable (light sensitive) in aqueous solution and self-degradable at night. This article covers curcumin's basic medicinal chemistry and stabilized it as it is an unstable, reactive, nonbioavailable chemical and, as a result, a very unlikely lead. It was necessary to stabilise the NC for use in treatment because NC suspension was only stable for 7 days at 4°C. Due to its sensitivity to pH, the colourant curcumin changes or loses colour when it encounters alkaline medium, such as dry powders (Figure 1).
Antiviral activity of curcumin
Finding new potent antiviral compounds is necessary due to the lack of effective treatments for many viral diseases, the advent of antiviral medication resistance, and the high cost of some antiviral therapies.3 Additionally, Moghadamtousi et al. found that the current antiviral medications are not always well-tolerated, quite effective, and satisfying.3 Consequently, the growing need for antiviral agents will be emphasised more. Scientists are interested in plants because they are a rich source of phytochemicals with diverse biological properties, including antiviral properties.4
Curcumin, a plant extract, has been shown to have antiviral effect against a variety of viruses. The inosine monophosphate dehydrogenase (IMPDH) enzyme has been recommended as a potential therapeutic target for antiviral and anticancer drugs due to its rate-limiting action in the de novo synthesis of guanine nucleotides. Due to its inhibitory efficacy against IMPDH in either a noncompetitive or competitive manner, the approach recommends curcumin as a strong antiviral component among the 15 different polyphenols.5 The investigation of various curcumin bioconjugates, such as di-O-tryptophanylphenylalanine curcumin, di-O-decanoyl curcumin, di-O-pamitoyl curcumin, di-O-bis-(γ,γ)folyl curcumin, C4-ethyl-O-folyl curcumin, and 4-O-ethyl-O-folyl cur Additionally, di-O tryptophanylphenylalanine curcumin and di-O-decanoyl curcumin, with EC50 values of 0.011 M and 0.029 M, respectively, showed considerable antiviral efficacy against VSV and FIPV/FHV. However, bioconjugates demonstrated no detectable antiviral effect in MT-4 cells against the HIV-1 type 1.6 IIIB and ROD strains. Table 1 outlines Curcuma longa and curcumin's antiviral activities, as well as the possible mechanisms underlying inhibitory effects.6–21
|
Virus |
Antiviral substances |
Description of antiviral activity type |
Reference |
|
HIV |
Curcumin, reduced curcumin, allyl-curcumin, and tocopheryl-curcumin are all types of curcumin. |
Inhibition of HIV-2 proteases, HIV-1 Integrase, Tat-mediated transactivation of HIV-1, HIV-1 LTR, and Tat protein acetylation |
|
|
Influenza |
Curcumin |
Inhibition of haemagglutination |
|
|
Coxsackievirus |
Curcumin |
Replication inhibition through UPS dysregulation |
|
|
HSV-1 |
Curcumin, gallium-curcumin, Cu-curcumin |
Reduction of HSV-1 replication |
|
|
HSV-2 |
Curcumin |
Significant protection in mouse model |
|
|
JEV |
Curcumin |
Reduction in production of infective viral particles |
|
|
HBV |
Aqueous extract |
Suppression of HBV replication by increasing the p53 level |
|
|
HCV |
Curcumin |
HCV replication is reduced by inhibiting the Akt-SREBP-1 pathway. |
|
|
HPV |
Curcumin |
Downregulation on HPV-18 transcription ; Inhibition expression of viral oncoproteins of E6 and E7 |
|
|
HTLV-1 |
Curcumin |
JunD protein is downregulated in HTLV-1-infected T-cell lines. |
Table 1 Antiviral activity of Curcuma longa L. and curcumin
Curcumin contains numerous clinically helpful properties, including anti-inflammatory, anti-arthritic, anti-cancer, antioxidant, antiviral, anti-ischaemic, and anti-amyloid properties. Curcumin has also been proven to have a high potential for reducing protein aggregation in severe diseases such as Parkinson's and Alzheimer's.
Activity against Coxsackievirus
Coxsackie viruses are enteroviruses that belong to the Picornaviridae family. Their small size (approximately 30 nm), lack of an envelope, and capsid icosahedral symmetry set them apart. Four structural proteins are found in the capsid: VP1, VC2, VF3, and FD4. The genome, according to Jacobs et al., is made up of 7.4 km2 of positively charged, linear RNA. Coxsackievirus B (CVB) includes six subtypes that cause myocarditis in both mice and humans.22 Cardiotropic coxsackievirus B subtype 3 (CVB3) is the main etiologic agent in viral meningitis and acute and chronic myocarditis, claim Ferreira et al. To combat the coxsackievirus, curcumin reduced viral RNA expression, protein synthesis, and virus titer. Additionally, it has been demonstrated to shield cells from cytopathic and virus-induced apoptosis.23
Activity against Murine Norovirus
Positive polarity single-stranded RNA genomes are present in the murine norovirus (MNV), family Caliciviridae.24 Human norovirus (HuNov), which is closely related to MNV and is the etiologic agent of acute gastroenteritis, is more relevant clinically. In addition to its considerable genetic variability, HuNov's high infectivity and environmental endurance make it crucial to battle.25 Since HuNovs cannot be regularly propagated, cultivable alternatives like feline calicivirus (FCV) and MNV are frequently utilised as experimental models to examine viral inactivation by bioactives in the management of gastrointestinal disorders.26
Curcumin has been proposed as a potential antinoroviral drug to control outbreaks of foodborne diseases.27 In another work, culturable FCVs and MNVs were used to study the effects of photoactivated curcumin. After incubation at 37°C for 30 min, photoactivated curcumin (50 g/mL) was found to decrease FCV titters by almost 5 logs. According to the authors, curcumin showed less antiviral activity against MNV under the same circumstances (decrease of 0.73 log TCID50/ml).28
Activity against Enterovirus 71 serotype (EV71)
The Enterovirus 71 serotype (EV71), family Picornaviridae, Enterovirus A species, primarily affects children, causing neurological and systemic difficulties as well as damage to the hands, feet, and mouth.29 Curcumin inhibited viral protein expression and RNA synthesis in vitro, demonstrating its antiviral activity against this virus.30
Activity against SARS-CoV and SARS-CoV-2
Coronaviruses (CoVs), members of the Coronaviridae family, are considered zoonoses of great medical concern since they may infect both humans and animals.31 They cause a wide range of respiratory infections, from the common cold to more serious conditions such as the Middle Eastern respiratory syndrome (MERS), Severe Acute Respiratory Syndrome (SARS), and coronavirus disease-2019 (COVID-19), which can be transmitted from person to person or from animal to person and cause a significant epidemic.32
When used as a dietary supplement, curcumin can promote treatment and prophylaxis for COVID-19 by boosting the immune system, halting the transmission of viruses, delaying the onset of severe disease, and further suppressing hyperinflammation.33 It can stop the replication of SARS-CoV-2 and repair COVID-19-related harm to pneumocytes, renal cells, cardiomyocytes, hematopoietic stem cells, and other cells.34 The clinical evaluation of curcumin in the therapeutic therapy of SARS-CoV-2 illness is supported by the published data.
Aqueous Chitosan-Tergitol-15-S-7 system for Curcumin nanosystem stabilisation
Binding of curcumin with chitosan: The highest absorbance of curcumin (2.510 mol dm3) in an aqueous solution with 25% MeOH occurs at pH 7.4 (phosphate buffer), at 425 nm. A shoulder and an absorption band are visible in aqueous curcumin at 365 and 425 nm, respectively. Curcumin's absorption band at 425 nm is created by the enol form, which predominates in both liquid and solid form, while the shoulder is brought on by absorption by a symmetrical structure with conjugation broken at the dike to groups, as shown in Scheme 1. At a pH of 7.4, Figure 2 demonstrates that larger chitosan concentrations lead to higher curcumin absorption intensities in the 425 nm band.35
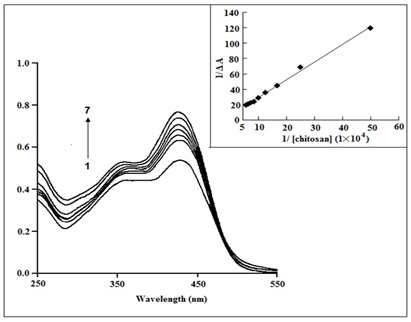
Figure 2 Curcumin (2.5×10−5 mol dm−3) absorption spectra at pH 7.4 in the presence of various concentrations of chitosan at 298(±0.1) K. [chitosan]: (1) 2µM, (2) 4µM, (3) 6µM, (4) 8µM, (5) 10µM, (6) 12µM, (7) 14µM. Inset: Plot showing the binding constant determination.35
Although the spectra of curcumin bound to chitosan are comparable to those obtained in the absence of chitosan, the intensities of the 425 nm band increase dramatically when chitosan is added, indicating that curcumin interacts with the polymer. Curcumin is mostly found in neutral form below pH 8.0 (Scheme 2).
The modified Benesi-Hildebrand equation, which is shown below, was used to measure absorbance changes at a suitable wavelength as a function of the assumption that chitosan and curcumin form a 1:1 combination.
(1)
Here, ΔA and Δε denotes the change in absorbance and molar extinction coefficient at the wavelength of the study (422 nm), respectively. [chitosan] and [curcumin] represent the chitosan and curcumin equilibrium concentrations, respectively.
At physiological pH levels, it is expected that the anionic component of curcumin will interact with the cationic polymer. In higher pH solutions (pH 6.5), the free amino groups of chitosan molecules become less protonated and the hydrophobic character throughout the chitosan chain becomes stronger. In phosphate buffer solutions, chitosan self-aggregates as a result of intra- and intermolecular hydrophobic interactions. The chitosan agglomerates that form could capture curcumin in its enol state. Furthermore, intermolecular hydrogen interactions between curcumin and the hydroxyl groups of chitosan's glucosamine unit may form. In addition to their hydrophobic interaction, curcumin and phosphatidylcholine have also been shown to interact via a hydrogen bond. By measuring the variations in curcumin-induced changes in chitosan absorption at 425 nm at various chitosan concentrations at pH 7.4 (phosphate buffer), varying the concentration of chitosan from 2M to 16M while maintaining the curcumin concentration constant at 25M, and fitting the data to the double reciprocal plot (Eq. (1)), it was possible to calculate the binding constant. Within this chitosan concentration range, the maximum of curcumin shifts slightly from 425 nm to 421 nm. As shown in Figure 2 (Inset), fitting the data to equation (1) yielded a linear curve with a squared correlation coefficient of 0.99, from which the binding constant was estimated to be 2.01(±0.5) ×104 M-1 at pH 7.4 and a system temperature of 298K (0.1).
Curcumin and chitosan binding has also been studied using fluorescence measurements.35 The medium has the greatest influence on curcumin fluorescence. Curcumin creates a very weak fluorescence band at 550 nm after illumination at 425 nm in aqueous buffer solutions containing 25% MeOH. The intensity of fluorescence increases considerably with a Stokes shift of about 80 nm in a hydrophobic macromolecular environment. However, when increasing the amount of chitosan is added to a fixed concentration of curcumin (2.5×10−5 M-1), the fluorescence spectrum becomes sharp and the fluorescence intensity significantly increases, with a slight hypsochromic shift from 550 nm to 539 nm due to curcumin binding with chitosan (Figure 3).
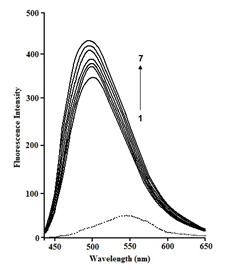
Figure 3 Fluorescence spectra of curcumin (2.5×10−5 mol dm−3) at pH 7.4 in presence of various concentrations of chitosan at 298 (±0.1) K. [chitosan]: (1) 2µM, (2) 4µM, (3) 6µM, (4) 8µM, (5) 10µM, (6) 12µM, (7) 14µM.35
As the microenvironment of curcumin was modified, a small spectrum shift in the em was noticed upon complexation of curcumin with chitosan. The dye is thought to live in the polymer's slightly nonpolar area, where the polarity and hence dielectric constant of the microenvironment are significantly lower than in bulk water. Following fluorescence intensity variations at 540 nm after excitation at 425 nm for solutions containing curcumin with various chitosan concentrations from 0.02mM to 0.2mM at pH 7.4 (phosphate buffer) at 298 (0.1) K, the binding constant for curcumin with chitosan was calculated. The binding constant K for the above-mentioned equilibrium (4) has been calculated to be 2.25(±0.5) ×104 M-1
Curcumin in chitosan-Tergitol-15-S-7 surfactant system
Figure 4 depicts the spectra of 2.5M aqueous curcumin generated by chitosan in the presence of 1.0 mM Tergitol-15-S-7 at pH 7.4 and 298 (0.1) K. In the presence of Tergitol-15-S-7, the intensity of the 420 nm band increases dramatically with increasing chitosan content. The binding constant of the curcumin non-ionic surfactant-polymer system was measured by measuring the change in absorbance values of aqueous curcumin at progressively increasing chitosan concentrations in a fixed concentration of Tergitol-15-S-7. The binding constant has been found to be 2.19(±0.5) ×105 M-1 which indicates that the curcumin-chitosan binding in the presence of Tergitol-15-S-7 is about ten times stronger than that in absence of the surfactant. This indicates that the dye and the chitosan-Tergitol-15-S-7 have a stronger hydrophobic contact. The positively charged polymer interacts electrostatically with Tergitol-15-S-7 in the chitosan-Tergitol-15-S-7 combination. The electronegative oxygen atoms of Tergitol-15-S-7's PEO chains may form associations with the electropositive chitosan chains. Excess polymer chains left behind after connection with Tergitol-15-S-7 may form a temporary network that can interact with curcumin. As a result, the hydrophobic interaction of curcumin with chitosan is more apparent in the presence of Tergitol-15-S-7.
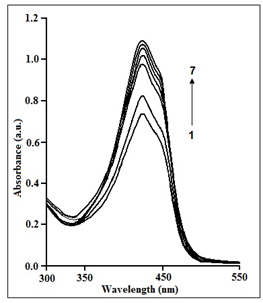
Figure 4 Curcumin (2.5×10−5 mol dm−3) absorption spectra at pH 7.4 at different doses of chitosan in the presence of 1×10−3 mol dm–3 TW80 at 298(±0.1) K. [chitosan]: (1) 2µM, (2) 4µM, (3) 6µM, (4) 8µM, (5) 10µM, (6) 12µM, (7) 14µM.
Fluorescence studies for aqueous curcumin were also performed in a non-ionic surfactant-chitosan system, and the results were used to estimate the binding constant. Aqueous curcumin containing 25% MeOH fluoresces more brightly as the amount of chitosan increases, with an effective blue shift from 550 nm to 490 nm in the presence of Tergitol-15-S-7. This considerable blue change was caused by a transition from a polar to a less polar habitat. Under physiological pH, the chitosan-Tergitol-15-S-7 combination is more successful at attaching curcumin molecules to the surfactant-free chitosan medium.
Curcumin and its analogues can inhibit the reproduction of numerous viruses in a variety of ways. Curcumin, on the other hand, has low bioavailability and is rapidly metabolised, limiting its effectiveness as an antiviral drug and likely contributing to its lack of success in human clinical studies. Furthermore, while high doses of curcumin appear to be safe in humans, most studies show in vitro CC50 values in the tens of micromolar, resulting in a relatively low SI, limiting its potential usefulness even further. Even at physiological pH, curcumin interacts strongly with chitosan, and the interaction is amplified in the presence of surfactants. The chitosan-curcumin binding constant was shown to be greater in the chitosan-Tergitol-15-S-7 combination than in the chitosan. Fluorescence quenching studies clearly reveal that one component of curcumin occupies the chitosan's hydrophobic interior, while the other fraction, anionic curcumin, occupies the polymer's cationic centres. Chitosan inhibits hydrolytic degradation of curcumin with a remarkable 75% yield. The yield increases to 95.5% when Tergitol-15-S-7 is added.
None.
None.
Author declares that there is no conflict of interest.

©2023 Baruah, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.