Journal of
eISSN: 2373-6453


Editorial Volume 10 Issue 2
1Faculty of Medicine, Western University, Thailand
210th Zonal Tuberculosis and Chest Disease Center, Thailand
3Department of Pathology, Faculty of Medicine, Chiang Mai University, Thailand
Correspondence: Attapon Cheepsattayakorn, 10th Zonal Tuberculosis and Chest Disease Center, 143 Sridornchai Road Changklan Muang Chiang Mai 50100, Thailand, Tel 6653140767, 6653276364, Fax 6653140773, 6653273590
Received: May 02, 2023 | Published: May 2, 2023
Citation: Cheepsattayakorn A, Cheepsattayakorn R, Siriwanarangsun P. COVID-19 vaccine induced t-cell immunity influenced by age and comorbidities. J Hum Virol Retrovirol. 2023;10(2):40-41. DOI: 10.15406/jhvrv.2023.10.00263
Severe acute respiratory syndrome-coronavirus type 2 (SARS-CoV-2) emerged in Wuhan, China in 2019 and caused coronavirus disease 2019 (COVID-19) and more than 1.4 million deaths, as of July 22, 2021.1 Severe form of infection is associated with respiratory distress syndrome, pneumonia, myocarditis, renal injury, gastrointestinal, testicular, ophthalmic, central-nervous-system diseases, etc.2 SARS-CoV-2, spherical form, diameter of 125 nm., and a single positive-strand-ribonucleic acid (RNA) is rapidly spread in animals and humans.3 S-protein of the surface of virus is most important for virus-host cell binding, fusion and host cell entry through the cellular Angiotensin Converting Enzyme 2 (ACE2) and finally infect the host cell,4 in addition to the sixteen non-structural proteins, and eight accessory proteins.5
In previous animal studies, protection from SAR-CoV-2 (COVID-19) infection contributed from both cellular and humoral immune responses. Most COVID-19 mRNA vaccine studies have concentrated on post-immunization-humoral-response characterization.6–10 Alpha, Delta variant strains, and original Wuhan strain contributes an association between protection against COVID-19 and antibody (humoral) level, detected by previous studies.11–13 With greater magnitude of CD4+ cells, compared with CD8+ T cells, persist-at-least-6-month-post-immunization-SARS-CoV-2-mRNA-vaccine-induced-cellular-immunity was evidenced by SARS-CoV-2-Spike-specific CD8+ and CD4+ T cells.14–16 Both cellular and humoral SARS-CoV-2 Spike Specific immune responses of the adaptive immune system rises with each vaccine dose, whereas progressively reduce with higher comorbidity prevalence and older age (Figure 1).17 A previous study demonstrated lower spike-protein antibody levels in individuals with medical conditions and those with 50 years of age and older in double Sino pharm vaccinated group, whereas a booster dose of Pfizer/BioNTechBNT162b2 vaccine critically increased spike-protein antibody levels.18
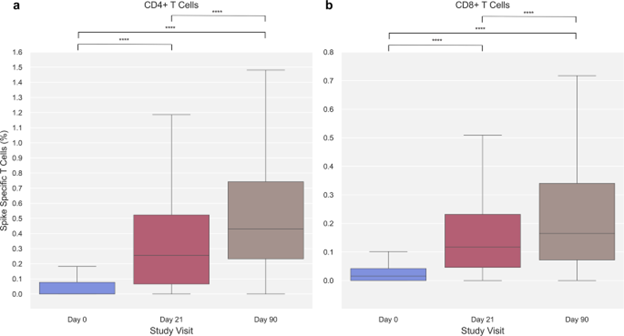
Figure 1
In conclusion, overall cross-reactive T cell responses against different SARS-CoV-2-variants of concern (VOC) in both previously infected and infection-naïve HCWs. For different VOC, COVID-19 booster vaccinations induce neutralizing antibody and strong T cell responses and the presence of T cell responses against SARS-CoV-2 VOC indicate that vaccine-induced T cell immunity contributes cross-reactive protection.
None.
None.
Author declares that there is no conflict of interest.

©2023 Cheepsattayakorn, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.