Journal of
eISSN: 2373-6453


Review Article Volume 8 Issue 2
School of Biotechnology,Gautam Buddha University,India
Correspondence: ImteyazQamar, Assistant Professor, School of Biotechnology, Gautam Buddha University Greater Noida, U.P.- 201312,India, Tel 91-120-2344280
Received: April 18, 2020 | Published: May 28, 2020
Citation: Banerjee P, Singh T, Singh N, et al. Comprehensive review on the evolution of SARS-CoV-2 (COVID-19): From emergence, outbreak, molecular characterization to the clinical challenges in designing and developing potential drugs, vaccines and therapies to counter SARS-CoV-2. J Hum Virol Retrovirolog. 2020;8(2):43-48. DOI: 10.15406/jhvrv.2020.08.00220
The outbreak of Coronavirus which initially began in China, in the year 2003 has caused several major public health concerns which resulted in epidemics globally, such as, severe acute respiratory syndrome (SARS) & Middle East respiratory syndrome (MERS). Most recently, i.e. in late December 2019, authorities identified an ongoing outbreak of the novel coronavirus (2019-nCoV), recently named as COVID-19 caused by SARS-CoV-2 which originated in Wuhan, Hubei Province, China. It probably marked the third highly pathogenic coronaviruses spreading globally, causing severe respiratory ailments and pneumonia-like infections in the humans.2019-nCoV exhibits similar genome organization, genetic, and epidemiological features to the other known CoVs. The COVID 19 virus has been declared as an inter-human transmissible virus which readily spreads mainly through the respiratory droplets produced when a person infected with coronavirus coughs, sneezes or talks. In a short stretch of time, this epidemic had spread to 210 Countries and Territories with 16, 20, 500 confirmed cases, including 97,400 confirmed deaths. Currently, the Mortality rate of COVID 19 is 2-5% only compared to SARS and MERS Coronaviruses. The older people are at a higher risk of COVID-19 infection due to their reduced immunity and body reserves, as well as multiple associated comorbidities like hypertension, diabetes, cancer and chronic obstructive pulmonary disorders. Till date, there is no effective anti-viral drug or licensed vaccine available to treat this infectious disease. As the infection is a recently advancing pathogen, several queries remain unanswered concerning the virus's reservoirs, pathogenesis, transmissibility, and much more is unknown. Herein, this article comprehensively reviews the currently available information on the virology, epidemiology, clinical presentation, diagnosis, and treatment of COVID-19.
Keywords: COVID 19, structural proteins, ACE 2, replication process, transmission, clinical features, diagnosis, antiviral drugs, vaccines
SARS, severe acute respiratory syndrome; MERS, middle east respiratory syndrome; 2019-nCoV, novel coronavirus; HR1, heptad repeats 1; HR2, heptad repeats 2
In 1930s, scientists identified a virus, later named as coronavirus which caused an acute respiratory infection in Domesticated chickens, later named infectious bronchitis virus (IBV). However, in the late 1940s, two more animal coronaviruses were recognized, namely, mouse hepatitis virus (MHV) which causes wide range of infectious disease in laboratory mice and transmissible gastroenteritis virus (TGEV) that infects pigs. Coronaviruses (CoVs) constitute an enormous group of viruses that are enveloped with a positive sense, single-stranded RNA genome and belongs to order Nidovirales, family Coronaviridae, subfamily Coronavirinae that are broadly distributed among birds, humans and other mammals. Coronaviruses typically result in severe infections, respiratory, enteric and systemic, affecting both humans and numerous animal hosts.1 The clinical disease termed COVID-19 was identified as an infectious disease caused by a newly recognized strain of beta coronavirus, now named as, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), introduced to humans for the first time. The emergence of novel Corona virus disease (COVID 19) which eventually originated in Wuhan, China has been identified as a deadly virus causing pneumonia like illness. 2 It has spread worldwide since then, growing into 2019-20 potential pandemic now. On 7th January 2020, the scientists from China worked on isolation of SARS-CoV-2 from an infected patient and conducted genome sequencing to get a better sense of the novel pathogenic virus.3 On 30th January 2020, the outbreak of COVID-19 was declared as the sixth Public health emergency of international concern (PHEIC), therefore this outbreak constitutes a public health risk through the international spread of disease and requires coordinated global response.
Etiology of COVID 19
Salient features of novel coronavirus: The family Coronaviridae which belongs to subfamily Orthocoronavirinae and order Nidovirales is further categorized into four genera of CoVs: α-CoV, β-CoV, γ-CoV, and δ-CoV. Viruses of genera α- and β-CoV are mostly known to infect mammals, γ-CoV causes infection in avian species while genus of δ-CoV have been found in both avian and mammalian species. Earlier, six Coronaviruses were identified as human-susceptible virus, among which α-CoVs includes: HCoV-229E and HCoV-NL63, and β-CoVs includes: HCoV-OC43 and HCoV-HKU1 which typically cause mild respiratory disorders.4 The other two previously known zoonotic β-CoVs emerged periodically in different areas, known as severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002 and Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012 which were considered to be much more fatal than 2019-n-CoV.All these human coronaviruses did not jumped from animals to human but rather utilized humans as a natural host. Zhu et al. found that the genome sequence of SARS-CoV-2 was phylogenetically similar to bat CoV RaTG13 with 96.3% similarity, whereas it shared 79.5% homology to SARS-CoV and 40% identity to MERS-CoV.3 Based on the results of genome sequencing and evolutionary analysis, bat has been suspected as natural host of virus origin, and SARS-CoV-2 might be transmitted from bats via unknown intermediate hosts to infect humans. It therefore appears that 2019-nCoV uses the same cellular receptor hACE2 (human angiotensin-converting enzyme 2) as SARS-CoV.5
SARS-CoV-2: Biology and replication machinery: The coronavirus owes its name due to the presence of crown-like Spike glycoproteins on their surface. Unlike other coronaviruses, SARS-CoV-2 is non-segmented, enveloped, positive-stranded RNA (+ssRNA) genome containing 29891 nucleotides, encoding for 9860 amino acids. As shown in Figure 1A & B, the SARS coronavirus contains upto 14 open reading frames (ORFs) expressed from the 3’ end of the viral genome, including 4 major structural proteins- envelope (E), membrane (M), spike (S), nucleocapsid (N) as well as non-structural proteins (nsp1-16) translated from open reading frames (ORFs) 1a and 1b including several accessory proteins, such as 3a,6,7a,7b and 8. 6 Among which, E has a critical role in virus pathogenicity which also promotes viral assembly and release. The S-glycoprotein is present on the surface of CoV and postulated to play a dominant role in viral attachment, fusion and entry into the host cell. The S protein consists of two subunits, i.e. S1 and S2. S1 determines the virus-host range and cellular tropism with the key function domain receptor binding domain (RBD) which strongly binds to the human ACE-2 (hACE-2) and bat ACE-2(bACE-2) receptors, whereas S2 mediates the cell membrane fusion by two tandem domains, Heptad repeats 1 (HR1) and Heptad repeats 2 (HR2).7 SARS-CoV-2 RBD exhibited higher binding affinity to ACE-2 receptor than SARS-CoV RBD hence could block the binding and attachment of SARS-CovV-2 RBD to hACE-2 expressing cells, thus prohibiting viral infection to host cells.7 Another recent research suggested8 that the mutation in NSP2 and NSP3 play a role in infectious capability and differentiation mechanism of SARS-CoV-2. This provokes people to explore the difference of the host tropism and transmission between SARS-CoV-2 and SARS-CoV or conduct further investigations on the potential therapeutic targets.
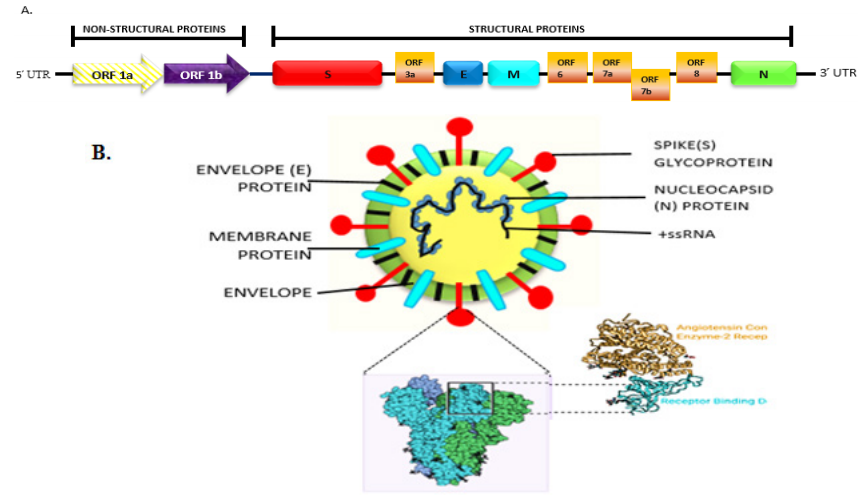
Figure 1 (A) Genomic organization of the ~30Kb Single-stranded RNA of SARS-CoV-2. (B) Schematic structure of the SARS-CoV-2 virion and its major structural proteins.
Replication process of COVID-19: It has been illustrated that SARS-CoV-2 possesses a similar replication mechanism to the SARS-CoV. Initially, the spike glycoproteins present on the surface of CoV binds to the cellular ligand ACE-2 receptor9 and causes activation of the complex by a type 2 transmembrane protease (TMPRSS2) (Figure 2A, B, C).10 The genome gets transcribed and translated once the virus makes its entry into the host cell. The replication and transcription processes occurs at the cytoplasmic membrane and involves coordinated procedures of both continuous and discontinuous RNA synthesis which are mediated by the replicase enzyme. Moreover, RNA-dependent RNA polymerase, RNA helicase and protease activities are most common to RNA viruses but recently the coronavirus replicase was predicted to employ a variety of RNA processing enzymes, that are not found in other RNA viruses and also includes the putative sequence-specific exonuclease, 2''-O-ribose methylnuclease, ADP ribose 1'' phosphatase and in a subset of group 2 coronaviruses, cyclic phosphodiesterase activities.1
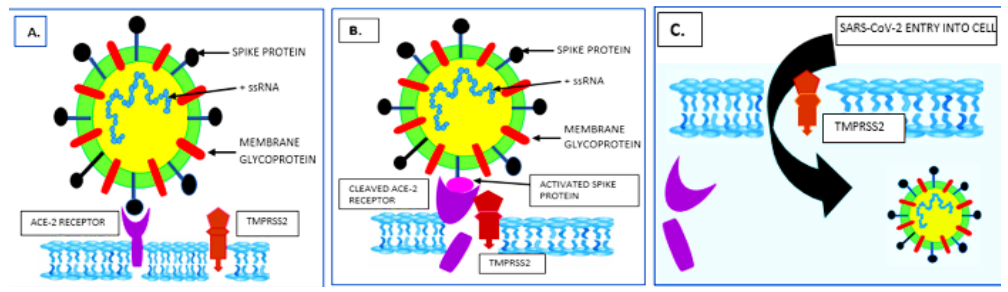
Figure 2 (A) Attachment and fusion of the Spike protein to the ACE-2 receptor on the surface of the target cell. (B) Cellular protease TMPRSS2 causing activation of the Spike protein by proteolytic cleavage (C) Cleaved ACE-2 receptor by its activated Spike facilitates the entry of SARS-CoV-2.
Origin, outbreak and transmission
The explosive outbreak of 2019-nCoV causing mysterious illness originated in China has been transmitted globally, probably by human movement and travel. Findings reported by the Health Commission of Hubei province, China, suggested that most of early infected patients were male with acute respiratory syndrome of unknown etiology who were linked to Huanan seafood wholesale market in Wuhan, China. However, there were 13 of the 41 cases had no link to the market place.6 Most importantly, in the earliest case, the patient suspected on 1st December 2019 had not any conveyed access to the seafood market, figure 3. The molecular findings did not suggest any no epidemiological association between the first patient and later cases. It seems that the seafood market is not the only origin of the virus. The virus probably came into the market place first then it went out of there.7 However, there is no solid evidence so far that the origin of SARS-CoV-2 was from the Wuhan seafood market which was taken as an indication of human-to-human transmission. Research is underway to elucidate more about etiology, transmissibility, severity, and different genetic features associated with COVID-19.
Many domestic and wild animals, including camels, cattle, cats, and bats, may serve as hosts for coronaviruses.8 It is considered that, usually, animal coronaviruses do not spread among humans3 and the novel coronavirus is of zoonotic origin. Rather, bats are the natural reservoir of a wide variety of coronaviruses, including severe acute respiratory syndrome coronavirus (SARS-CoV) like viruses. SARS-CoV-2 originated from Miniopterus bats or unknown intermediate hosts and crossed the species barrier into humans, Figure 4. The latest guidelines from WHO described three main transmission routes for the COVID-19: 1) droplets transmission, 2) contact transmission, and 3) aerosol transmission. Droplets transmission was reported to occur when respiratory droplets (as produced when an infected person coughs or sneezes) are ingested or inhaled by individuals nearby in close proximity; contact transmission may occur when a person touches a surface or object contaminated with the virus and subsequently touch their mouth, nose, or eyes; and aerosol transmission may occur when respiratory droplets blend into the air, forming aerosols which may cause infection when inhaled high dose of aerosols into the lungs in a relatively closed environment.
Clinical manifestations
On the basis of present scientific investigation, most individuals had a history of close contact to a patient who had been infected with COVID-19 or a history of travel from Wuhan City or Hubei province, China. Most estimates of the incubation period generally varies from 3–14 days after infection.9
As reported COVID-19 is transmissible among humans, especially the older adults and people of any age with pre-existing health conditions (such as-diabetes, cardiovascular disorder, liver disorder, cancer or chronic lung disease) are more susceptible and prone to illness.10 According to a new study, the risk of death from COVID-19 is only 1.38%. However, the mortality rate rises with age, from well below 1% among children aged 9 years or younger to nearly 8% for seniors aged 80 years or older. The median age of patients is 47–59 years, and 41.9–45.7% of patients were females.
Signs and symptoms: Based on the current reports, people infected with the virus may develop symptoms from mild to severe pneumonia like illness leading to death. The most common symptoms of novel coronavirus are fever, tiredness, dry cough and dyspnea,11 whereas in few patient’s non-respiratory symptoms such as headache, muscle ache, rhinorrhoea, sneezing, sore throat and phlegm-producing cough, diarrhoea and nausea have also been reported. The disease tends to progress faster in the elderly and those with underlying disorders (i.e., hypertension, chronic obstructive pulmonary disease, diabetes, cardiovascular disease). Once infected, these people may rapidly develop acute respiratory distress syndrome (ARDS), septic shock followed by metabolic acidosis hard to correct and coagulation dysfunction.
Diagnostic criteria
Diagnostics are essential weapons in our arsenal against the emerging pathogenic threats. A critical shortage in testing components for SARS-CoV-2 is posing its drastic effects in several parts of the Country, preventing people from accessing care. Diagnostic testing has highlighted the importance of COVID-19 to understand the epidemiology, case management and transmission. Different countries have adopted different testing strategies, reflecting the availability of diagnostics and reagents, and the needs of their individual health systems. A wide range of diagnostic tests are commercially available for SARS-CoV-2, some of which have been received by different national and other regulatory agencies. Based on the clinical and epidemiological factors, the doctors decide whom to test.
Laboratory diagnosis can be performed by: (a) detecting the genetic material of the virus, (b) detecting the antibodies that neutralize the viral particles of interest, (c) detecting the viral disease on the basis of signs and symptoms (Table 1). For SARS-CoV and MERS-CoV, specimens collected from the lower respiratory tract such as sputum and tracheal aspirates have higher and more prolonged levels of viral RNA because of the tropism of the virus. However, the most effective and efficient decentralized screening method is real time reverse transcription polymerase chain reaction (Rrt-PCR) that is used to detect the positive nucleic acid of SARS-CoV-2 in sputum, throat swabs, and secretions of the lower respiratory tract samples.12
|
Types of diagnostic tools |
||||
|
Molecular assay |
Immunoassay |
Non-disease specific tests |
||
|
Antibody based |
Antigen based |
|||
|
Working |
It is useful in the detection of viral genetic material in the sample |
It helps in the detection of anti-viral antibodies in the sample |
It helps in the detection of viral proteins (antigens) in the sample |
Detection of the disease on the basis of signs and symptoms |
|
Techniques involved |
Real time reverse transcription polymerase chain reaction (Rrt-PCR) is globally used to produce millions of copies of a specific fragment of a viral genome and amplify minute amounts of detectable levels |
Enzyme-linked immunosorbent assay (ELISA) is commonly used to detect and measure the antibodies or antigens in the sample |
Other methods include point-of-care (POC) testing devices, thermal scanning used for the identification of individuals with fever(higher than normal temperature) and computer tomography (CT) chest scans/ radiographs to distinguish from other chest infections |
|
|
Uses |
Testing people suspected of having COVID-19 |
Assessing the overall infection and immunity rates in a community |
Testing people suspected of having COVID-19 or screening to identify suspects for further testing (depending on test design) |
Screening to identify the suspected cases for further testing |
|
Confirmation of present sars-cov-2 infection |
100% confirmation of recent SARS-CoV-2 |
Indication of current or past infection and could be used to screen for current infection (tests may not be reliable in early phases of infection) |
Confirmation of SARS-CoV-2 infection/suggests a potential infection (depending on test design) |
Suggests a potential infection and indicates that further testing is needed |
Table 1 Standard Laboratory Tests for Diagnosis of SARS-CoV-2
Perspectives on treatment strategies
Currently, there are no vaccines or antiviral medicines specific to the new coronavirus. The best preventive measure is to avoid being exposed to the virus by maintaining social distancing as advised by WHO. Treatment for now focuses on relieving symptoms such as breathing assistance. Pharmaceutical Companies across the world are racing to develop potential vaccines. A few have launched early safety testing in humans, but since the efforts to design and develop any vaccine or antiviral agent to tackle the presently emerging coronavirus pathogen would take some time, therefore till then we need to rely extensively on enforcing highly effective prevention and control measures to minimize the risk of COVID-19 transmission and spread awareness to the best feasible extent.13 Some scientists have already identified subtle changes since SARS-CoV-2 emerged in Wuhan, China, in December. But recent studies show the virus is relatively stable, which suggests vaccines should still be effective when they become available. Hence, efforts are being made to develop S-protein based vaccines to generate long-term and potent neutralizing antibodies or protect immunity against SARS-CoV-2. Here are some early treatment options by using antimicrobials and neuromicrobials inhibitors to protect patients with severe acute respiratory illness against SARS-CoV-2. However, many of these therapies are not specifically designed to treat COVID-19, but rather to treat diseases with similar symptoms to COVID-19.
Potential antiviral medications
However, many of these treatments were not specifically designed to treat COVID-19, but rather to treat diseases with similar symptoms to COVID-19 in the past. Several drugs such as ribavirin, penciclovir, arbidol nitazoxanide, nafamostat and favipiravir (T-705) are proposed and tested in vitro for their efficacy and treatment of COVID-19.23 Amongst the mentioned drugs, ribavirin, penciclovir, and favipiravir require high concentration of nucleoside analogs to reduce the viral infection and are considered less effective. The two antiprotozoal agents, Nafamostat and nitazoxanide are capable of preventing membrane fusion, hence exhibits inhibitory activity against COVID-19.
Potential vaccines
In recognition to the complex molecular make-up and other intriguing mechanisms of SARS-Cov-2, Major efforts now turn to the development of vaccines. Many years of extensive research failed to develop any effective vaccine against human CoVs but majority of the vaccines that have been developed for coronaviruses targets the Spike glycoprotein or S protein.25 This is mainly because of the fact that S protein is the major inducer of neutralizing antibodies .Several kinds of vaccines based on S protein have been previously studied. Among them, the S protein-RBD based vaccines include full length S protein vaccines that has been widely proposed and are already under clinical trials.7 mRNA-based vaccine are also under the development which converts the SARS-CoV-2 spike protein sequence into messenger RNA(mRNA).This type of vaccine is anticipated to cause initiation of the lymphatic immune cells to process the coded mRNA and in turn produce proteins in a manner where other immune cells recognize and begin mounting relevant response to acute infection,26 DNA-based vaccine for COVID-19 that rely on Spike proteins have also been closely studied from the progressive development of MERS-CoV vaccine. The proposed vaccine with targeted viral DNA sequence is expected to increase the immunity to fight against the viruses.27
Even though such therapeutic options have proven efficacy in the in vitro studies, however most of these haven’t undergone randomized animal or human trials and hence are of limited use in our present 2019-nCoV scenario.
The review focuses on a holistic picture of the current research going on in response to the outbreak of contagious COVID-19, which led to a medical emergency worldwide. Over the past decades, a mysterious outbreak of several life threatening human pathogens were observed, namely- Ebola, zika, nipah, SARS-CoV, MERS-CoV and the most recently evolved SARS-CoV-2, now named as COVID-19 has spread across the world causing pandemic after its emergence from the Chinese City Wuhan in late 2019 .After its origin from China, the new virus has infected nearly 16,20,300 people across the globe, a milestone just four months after its first outbreak. The death toll has exceeded over 98,000 with 3, 65,000 people recovered. A team of scientists in Shanghai sequenced the complete genome and through a phylogenetic comparison found it be 96% similar to the SARS-CoV which emerged in the year 2003 in bats. The physiochemical properties of the virus is yet to be focused on but according to a recent study SARS-CoV-2 possess similar characteristics to the other two known coronaviruses. A wide range of diagnostic tests are available commercially, but at present polymerase chain reaction (PCR) and antibody testing are the dominant ways that global healthcare systems are testing patients for COVID-19. Still there is a looming shortage in lab diagnostics which is a threating to delay coronavirus test results and has created a major gap in public health response. While a therapeutic strategy is being outlined here, the long-term goal of the novel COVID-19 research would remain developing an effective vaccine to yield neutralizing antibodies, likely based on the more specific RBD protein. But, the pandemic has catalyzed the development of COVID-19 vaccines across the biotech industry, both by pharmaceutical companies and research organizations, challenging the economic, medical and public health infrastructure throughout. At present, it’s necessary to implement the appropriate and control measure that are continuously being suggested by the government and public health authorities and prevent the spread of the disease.
None.
The author declares no conflicts of interest.

©2020 Banerjee, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.