Journal of
eISSN: 2373-6453


Review Article Volume 3 Issue 6
1Department of Microbiology, Ekiti State University, Nigeria
2Department of Microbiology, Federal University of Technology Akure, Nigeria
3Department of Microbiology, Ekiti State University, Nigeria
4University Health Centre, Ekiti State University, Nigeria
4Department of Microbiology, College of Medicine, Hallym University, South Korea
Correspondence: Oloruntuyi Adedayo Blessing, Department of Microbiology, School of Science. Federal University of Technology Akure, Nigeria, Tel +2348067338756
Received: August 13, 2016 | Published: October 14, 2016
Citation: Olubukunola OA, Blessing OA, Moses DO, Olawale EC, Adekunle BJ (2016) Antibiotic Susceptibility and Carriage Rate of Salmonella Serotypes among Healthy Individuals with History of Salmonella Infection within One Year in a University Community in Nigeria. J Hum Virol Retrovirol 3(6): 00113. DOI: 10.15406/jhvrv.2016.03.00113
The increase in the rate of carrier of typhoid fever is on the increase in the recent time and this present a serious threat to the public health. Students in most cases are living in densely populated hostels with poor sanitary conditions. This coupled with poor hygienic practices constitute pre-disposing factors. This study investigates the carriage rate and antibiotic resistance profile of Salmonella among students in a tertiary institution who had suffered gastroenteritis and typhoid fever within one year of infection. Seventy four (74) stool samples were obtained from students in the university. Isolates were identified using standard methods, subjected to antimicrobial susceptibility by disc diffusion method. The incidence of the infection was found to be highest among participants of 20 - 24 years age group. Fifty-one (51) faecal samples were positive for enteric pathogens. Six (11.76%) Salmonella species were isolated from stool culture and all were Salmonella paratyphi B serotype. All the Salmonella paratyphi B showed hundred percent (100%) resistance to Ceftazidime, Ampicillin, Amoxicillin clavulanic acid and Cefuroxime but were susceptible to Ofloxacin and Ciprofloxacin. High carriage of Salmonella observed from this study calls for proactive action since most of the isolates were resistance to commonly prescribed antibiotic drugs.
Keywords:Salmonella, Salmonellosis, Antimicrobial resistance, Gastroenteritis, Typhoid fever
Salmonella infection commonly refers to as Salmonellosis is an infectious disease of humans and animals caused by organisms of the two species of Salmonella (Salmonella enterica, and Salmonella bongori). Globally, Salmonella enterica serotypes causes up to 27 million infections occur per year, with over 2x105 attributable deaths annually, predominantly among children under the age of five years.1 Within this genus, more than 2,500 serovars have been described.2,3
Although all serovars may be regarded as potential human pathogens but majority of the infection is caused by few serovars. Salmonellosis is an important health problem and a major challenge worldwide having greater significance in developing countries.4 It also contributes to negative economic impacts due to the cost of surveillance investigation, treatment and prevention of illness.5
Salmonella organisms are aetiological agents of diarrhea and systemic infections in humans. Enteric fever (typhoid or paratyphoid fever) is a systemic infection caused by several Salmonella enterica serotypes including Salmonella typhi and Salmonella paratyphi A, B, or C. Salmonella enterica serovar typhi (S. typhi) is a human restricted pathogen.6,7 The incidence of typhoid fever remains very high in impoverished areas and the emergence of multidrug resistance had made the situation worse.8 This group of microorganisms adapts to a wide range of foods, becomes endemic and causes high morbidity with a wide spectrum of clinical manifestations such as diarrhea, nausea, abdominal cramps, vomiting and fever.5 Transmission is by the faecal-oral route whereby the intestinal contents of an infected animal are ingested with food or water. Human carriers are generally less important than animals in transmission of Salmonella strains. Meat, poultry products, dairy products, and fruits and vegetables are primary transmission vehicles; they may be undercooked, allowing the Salmonella strains to survive, or they may cross-contaminate other foods consumed without further cooking.9
Following oral uptake, Salmonella are exposed to stressful environments such as low pH, antimicrobial effect of bile, decreasing oxygen supply, normal gut flora and metabolites, cationic antimicrobial peptides present on the surface of epithelial cells. Salmonella invades a host cell by delivering into the cytoplasm virulence factors which directly interact with host regulators of actin polymerization which leads to bacterial uptake. It biosynthesizes a virulence capsular polysaccharide named as Vi antigen. Moreover, Salmonella avoids vacuole lyses and modulates the early and late endosomal markers presented in the vacuole membrane.10
Salmonellosis becomes endemic and causes high morbidity with a range of clinical manifestations such as diarrhea, nausea, abdominal cramp, vomiting and fever.11 The prevalent Salmonella infection is typhoid fever, caused by Salmonella typhi, which is responsible for life threatening infections in resource-poor nations. However, the true magnitude of salmonellosis is difficult to quantify because the clinical picture is confused with many other febrile illnesses and most typhoid endemic areas lack facilities to confirm the diagnosis.12
Beta-Lactams and fluoroquinolones are generally used to treat invasive Salmonella infections, but emergence and spread of antibiotic-resistant strains are being increasingly notified in many countries. In particular, detection of extended-spectrum β-lactamases (ESBLs) in Salmonella serotypes is a newly emerging threat worldwide.13 Increasing occurrence of antimicrobial resistance in both typhoidal and nontyphoidal Salmonellae is a serious public health problem. This aims and objectives of the study are, to isolate and characterize Salmonella serotypes among the healthy carriers in the University community, to determine the magnitude and know the circulating serotype of Salmonella serotypes among the students and to determine the level of antibiotics resistance of the serotype isolated from the healthy carriers.
Study design and period
A prospective cross-sectional study was conducted to determine the carrier rate of Salmonella serotypes among the healthy individual who had recovered from Salmonella infections between January 2010 to November 2011.
Study area
The areas for this study were the Ekiti State University community which includes, Student hostels, Iworoko - Ekiti and Ado-Ekiti. Students from the nine faculties in the University were involved in the study (Figure 1).
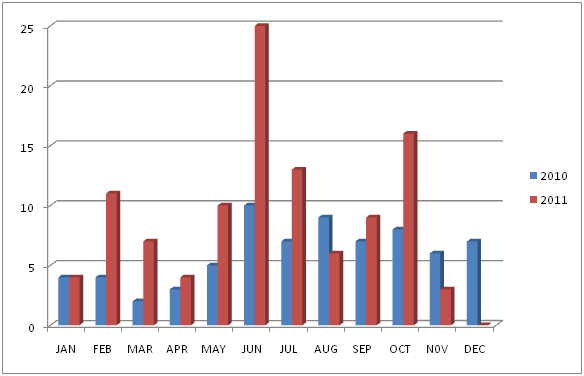
Figure 1 Occurrence of Salmonella infection between 2010 and 2011 in the study area.
JAN: January, FEB: February, MAR: March, MAY: May, JUN: June, JUL: July, AUG: August, SEP: September, OCT: October, NOV: November, DEC: December.
Study subjects and population
A total of 1,000 questionnaires were administered across the faculties among the students who were diagnosed at the University Health Centre of gastroenteritis and those who had typhoid fever. Only subjects that gave verbal consents were included in the study. Inclusion criteria were as follows: individuals who were students of the University, regardless of their ages across the faculties in the university, those who were reportedly to be ill of Salmonella infections between January 2010 to November 2011 at the university health centre and those who indicated that they had recovered from the Salmonella infections.
Collection, handling and culture of specimens
Freshly passed faecal specimens were collected and 1g of the faeces with a sterile wood spatula was transferred and pre enriched into prepared buffered peptone water (1part sample + 9 part buffer) in test tube. Mixed and incubate at 37° C overnight (16-20 hours).14 Transferred of approximate 1ml of the pre-enrichment was pipetted into 10ml Rappaport-Vassiliadis soy peptone (RVS) broth and incubated tube at 41.5° C overnight (18-24 hours).14 A loop full from the inoculated and incubated RVS broth were emulsified at a point and streaked out on Bismuth Sulphite Agar and incubate at 37° C overnight (18 - 48 hours). Brown and black colonies growths on plate were picked and subject to biochemical test. Identification of Salmonella serotypes was done biochemically as describe by.15
For the enumeration of other enteric pathogens present in the stool, the samples were differently cultured on MacConkey agar and incubated at 37° C for 24h. Representative colonies were subculture and identified using biochemical and cultural characteristics of the organisms.
Serotyping of Salmonella isolates
Serologic identification of Salmonella serotypes were performed using Wellcolex colour Salmonella test kit. Suspected Salmonella colonies from the culture plate were carefully emulsified in 200µl of sterile normal saline in a suspension tube. Holding the bottle vertically, re-suspended latex reagent 1 and 2 were dropped into a separate circle on a flat reaction card after shaking vigorously for few seconds. Transferred of approximately 40µl of bacterial suspension to two of the reaction circles containing latex reagent 1 and 2 respectively and mixed. Placed card on a suitable flatbed rotator and run at 150 ± 5 rpm for 2 minutes then switch off and observed for agglutination without removing the card rotator. Positive controls with the positive control reagents (green, blue and red control) were carried out alongside with the latex reagent 1 and 2 respectively without the inoculums. Results are then interpreted according to the manufacturer guidelines for usage of the kit.
Antimicrobial susceptibility of the Salmonella isolates
The isolates were standardized according to Bauer et al.16 and the antibiotic susceptibility testing of Salmonella serotypes was performed using the disk diffusion method, the zone of inhibition was measured to the nearest millimeter and results were interpreted according to Clinical and Laboratory Standards Institute.17 The antibiotics used were obtained from Abtek Biological Ltd, UK with the following concentrations in Kg: ceftazidime (CAZ) (30Kg), cefuroxime (CRX) (30Kg), gentamicin (GEN) (10Kg), ciprofloxacin (CPR) (5Kg), ofloxacin (OFL) (5Kg), amoxicillin/clavulanic acid (AMX/CLAV) (30Kg), nitrofurantion (NIT) (300Kg) and ampicillin (AMP) (10Kg) were tested against the isolates. Multiple antibiotics resistant was determined as resistant to three or more classes of antibiotics tested.
A total of 1000 questionnaire were administered randomly across the nine (9) faculties in the University out of which 826 were filled correctly and 174 were voided during the study. Out of the 826, 180 indicated positives Salmonella infections (typhoid and gastroenteritis) within January, 2010 to November 2011. While 646, claimed not to have been diagnosed of the infection. The age and sex of the 180 students who had recovered having been diagnosed of gastroenteritis and typhoid fever is presented in Table 1. The occurrence of gastroenteritis was highest among those who were within the age range 20 - 24 who had 107 (59.44%), then 25 - 29 had 45 (25.00%), 15 - 19 had 24 (13.33%) and 30 - 34 had 4 (2.22%). High prevalence of the infection was found in female with 112 (62.22%) students and male 68 (37.78%) students. The variation of occurrence of typhoid fever and the incidence of gastroenteritis within January 2010 and December 2011 among the 180 students showed that the peak of occurrence was in the month of June which coincides with the rainy season as presented in Table 2.
Sex |
Age (Years) |
||||||
15-19 |
20-24 |
25-29 |
30-34 |
35-39 |
Total |
% |
|
Male |
4 |
38 |
22 |
4 |
0 |
68 |
37.78 |
Female |
20 |
69 |
23 |
0 |
0 |
112 |
62.22 |
Total |
24 |
107 |
45 |
4 |
0 |
180 |
100 |
Percentage |
13.33 |
59.44 |
25.00 |
2.22 |
0 |
100 |
|
Table 1 Age and sex distribution of subjects participated in the study
Months |
|||||||||||||
Year |
Jan |
Feb |
Mar |
Apr |
May |
Jun |
Jul |
Aug |
Sep |
Oct |
Nov |
Dec |
Total |
2010 |
4 |
4 |
2 |
3 |
5 |
10 |
7 |
9 |
7 |
8 |
6 |
7 |
40 |
2011 |
8 |
15 |
9 |
7 |
15 |
35 |
20 |
15 |
16 |
24 |
9 |
7 |
60 |
Percentage |
4.4 |
8.3 |
5.0 |
3.8 |
8.3 |
19.4 |
11.1 |
8.3 |
8.8 |
13.3 |
5.0 |
3.8 |
100 |
Table 2 Seasonality of occurrence of Salmonella infections among the students examined
Jan: January; Feb: February; Mar: March; May: May; Jun: June; Jul: July; Aug: August; Sep: September; Oct:
October; Nov: November; Dec: December
The Widal test result of the students examined is presented in Table 3. One hundred and twenty seven (127) (70.56%) did not know their titre result, 23 (12.78%) had a titre of 1/80, 24 (13.33%) had 1/160, 4 (2.22%) had 1/320 and 2 (1.11%) had 1/640.
Titre |
1/80 |
1/160 |
1/320 |
1/640 |
Total |
Frequency |
23 |
24 |
4 |
2 |
180 |
Percentage |
12.78 |
13.33 |
2.22 |
1.11 |
100 |
Table 3 Titre values of the Widal test conducted on the subjects
Out of the 180 students who had been diagnosed of gastroenteritis and typhoid fever only 74 students whose consent were obtained gave out their stool sample. Fifty one (51) faecal samples of the stool submitted showed growth on Bismuth Sulphite agar between 24 - 48 hours of incubation while the remaining 21 showed no growth after this period of incubation.
The profile of bacterial pathogens isolated at from the stool of the subjects is presented in Table 4. The pathogens isolated were Klebsiella spp 15 (29.41%), Proteus spp 10 (19.61%), Salmonella serotype 6 (11.76%), Escherichia coli 5 (9.80%), Morganella spp 3 (5.88%), Citrobacter spp 2 (3.92%) while Shigella spp, Edwardsiella spp, Yersinia spp had one isolate each and six were unidentified after the biochemical test.15
Organisms Isolated |
Frequency |
% |
Salmonella serotype |
6 |
11.76 |
Proteus spp |
10 |
19.61 |
Shigella spp |
1 |
1.96 |
Escherichia coli |
5 |
9.80 |
Klebsiella spp |
15 |
29.41 |
Citrobacter spp |
2 |
3.92 |
Molrganella spp |
3 |
5.88 |
Edwardsiella spp |
1 |
1.96 |
Providencia spp |
1 |
1.96 |
Yersinia spp |
1 |
1.96 |
Unidentified |
6 |
11.76 |
Total |
51 |
100 |
Table 4 Occurrence of bacteria pathogens isolated from stool of subjects with typhoid and gastroenteritis
Spp: Species
Serologic testing with Wellcolex Salmonella test kit showed that the six Salmonella spp isolated were Salmonella paratyphi B as presented in Table 5. There was red agglutination of the organisms with both latex reagent 1 and 2. The agglutination in reagent 2 showed the presence of virulent (Vi) antigen in the Salmonella serotypes isolated.
Isolate |
Agglutination |
Colour |
Salmonella serotype |
Antigen |
||
|
Reagent 1 |
Reagent 2 |
Reagent 1 |
Reagent 2 |
|
|
1 |
+ |
+ |
Red |
Red |
S. paratyphi |
Vi |
2 |
+ |
+ |
Red |
Red |
S. paratyphi |
Vi |
3 |
+ |
+ |
Red |
Red |
S. paratyphi |
Vi |
4 |
+ |
+ |
Red |
Red |
S. paratyphi |
Vi |
5 |
+ |
+ |
Red |
Red |
S. paratyphi |
Vi |
6 |
+ |
+ |
Red |
Red |
S. paratyphi |
Vi |
Table 5 Serogroup of Salmonella isolated from the students examined
Positive (+); Negative (¬)
The percentage resistances of the bacterial pathogens isolated from the 51 students examined were having 100% resistance to ampicillin, amoxicillin/clavulanic acid and cefuroxime while ciprofloxacin and ofloxacin were susceptible with one or two resistances to them as presented in Table 6. Gentamicin, nitrofurantion and ceftazidime had variable resistance percentage. The resistance patterns of the Salmonella paratyphi B isolated is presented in Table 7. All were resistance to ceftazidime, ampicillin, Augmentin and cefuroxime. Two of the S. paratyphi B had resistant pattern CAZ/AMP/AMX-CLAV/CRX. The entire organisms isolated were having multiple resistance patterns to the class of cephalosporin, penicillin, aminoglycoside and macrolides.
Antibiotics |
Pathogens |
|||||
|
Salmonellaspp (n=6) |
Proteus spp (n=10) |
E. coli (n=5) |
Klebsiella spp (n=15) |
Citrobacter spp (n=2) |
Morganella spp (n=3) |
CAZ (%) |
6 (100) |
9 (90) |
4 (80) |
15 (100) |
2 (100) |
3 (100) |
AMP (%) |
6 (100) |
10 (100) |
5 (100) |
15 (100) |
2 (100) |
3 (100) |
NIT (%) |
3 (50) |
3 (30) |
4 (80) |
7 (46.67) |
0 |
1 (33.33) |
AMX/CLAV (%) |
6 (100) |
10 (100) |
5 (100) |
15 (100) |
2 (100) |
3 (100) |
OFL (%) |
2 (16.67) |
0 |
0 |
2 (13.33) |
0 |
1 (33.33) |
CIP (%) |
0 |
0 |
0 |
2 (13.33) |
0 |
1 (33.33) |
GEN (%) |
2 (33.33) |
5 (50) |
2 (40) |
2 (33.33`) |
0 |
1 (33.33) |
CRX (%) |
6 (100) |
10 (100) |
5 (100) |
15 (100) |
2 (100) |
3 (100) |
Table 6 The percentage resistance of the bacterial pathogens isolated from the stool of the subjects
CAZ: Ceftazidime; AMP: Ampicillin; GEN: Gentamicin; CRX: Cefuroxime; NIT: Nitrofurantion; AMX/CLAV:
Amoxicillin/Clavulanic acid; OFL: Ofloxacin; CIP: Ciprofloxacin; n: Number
Resistance pattern |
Frequency |
CAZ, AMP, GEN, CRX |
2 |
CAZ, AMP, NIT, AMXCLAV, CRX |
2 |
CAZ, AMP, AMX/CLAV, CRX |
1 |
CAZ, AMP, NIT, AMXCLAV, OFL, CRX |
1 |
Table 7 Antibiotics resistance pattern of Salmonella serotype isolated
CAZ: Ceftazidime; AMP: Ampicillin; GEN: Gentamicin; CRX: Cefuroxime; NIT: Nitrofurantion; AMX/CLAV: Amoxicillin/Clavulanic acid; OFL: Ofloxacin
Epidemiological investigation of salmonellosis and carriage rate in developing countries like Nigeria is difficult because of the very limited scope of the studies and lack of coordinated surveillance systems. Out of the 1000 questionnaire administered in this study, 180 (18%) were positive for Salmonella infections with high symptoms of headache, fever, weakness, vomiting, loss of appetite, stooling and cold.
Female were more susceptible to the infection than the male counterpart with 122 (62.22%) and 68 (37.78%) respectively in this study, which is in contrast to similar findings by Okonko et al.18 but Zailani et al.19 found no influence of age and sex on the distribution pattern of Salmonella species in Ile - Ife.
The highest occurrence of Salmonella infections were found in 20 - 24 years age group from the participant studied. This also agrees with Liilian et al.20 which find a significant prevalence of Salmonella infection within this age group. Mengistu et al.21 also reported that Salmonella were more prevalent among adult age greater than 15 years.
Akinyemi et al.22,23 saw in his study in Nigeria that typhoid fever was prevalent during the wet season. This study also shows that highest prevalence of the infection was found within spring and summer seasons (May and October) usually referred as the rainy season with the peak of the disease occurring in June. Typhoid fever is water and food borne disease, and an average temperature of 35° C during the rainy season provides the optimum conditions for the growth of bacteria. Similar findings in India, Shina et al.24 also found highest incidence of typhoid in the monsoon season.
The Widal test is rapid and sensitive in the diagnosis of Salmonella infections (typhoid and paratyphoid). 16.66% were having active infections with the significant of 1/160 and above as presented in Table 3, this significant is in line with Udeze et al.25 and Liilian et al.20 in diagnosis of asymptomatic students at Ilorin. 127 (70.56%) knew not their titre result and this can increase the spread of the infection due to their ignorance about the titre results.
In the present study a total of 51 entropathogens were isolated from stool (Table 4). The overall prevalence of Salmonella in this study was 8.11% (six Salmonella serotypes) this also conformed to the work of Abdullahi, 2010 in Kano State with the incidence of 13.50% also with the work of Cajetan et al.26 with prevalence of 2.3% out of 400 samples.
The isolates resistant to four or more separate classes of antimicrobials were defined as multidrug resistant.27 The incidence of resistance (i.e. resistance to two drugs) and multidrug-resistance (i.e. resistance to four or more drugs) of all Salmonella strains isolated is presented in Table 6. Salmonella sensitivity was highest to ciprofloxacin (100%) and ofloxacin (83.33%) similar to Bahnass et al.28 which reported that Salmonella strains were susceptible to ciprofloxacin likewise observed by Cajetan et al.26 The sensitivities of the other antibiotics were as follows: gentamicin (66.67%) and nitrofuratoin (50%) while cefuroxime, amoxicillin/clavulanic acid, ampicillin, and ceftazidime were resistant to by the Salmonella species isolated.
Conclusively, the high prevalence of salmonellosis needed to be monitored since most of the species isolated were resistance to most of the commonly available antibiotics and also the provision of portable water for drinking should be at its climax since the organisms is mainly transmitted by water.29,30 The epidemiology of salmonellosis in Nigeria had to be well explored since the serotypes and resistance pattern of the isolates may vary greatly in different geographical areas and there is a need for the development of guidelines for antibiotic treatment in this area.
The authors thanks the technical staff of the Department for the assistance rendered during the course of this work.
None.

©2016 Olubukunola, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.