Journal of
eISSN: 2373-6453


Review Article Volume 8 Issue 4
1Phytopathology and Microbial Biotechnology, Department of Botany, Mohanlal Sukhadia University, India
2Department of Botany, Acharya Narendra Dev College, University of Delhi, India
Correspondence: Mukesh Meena, Laboratory of Phytopathology and Microbial Biotechnology Department of Botany, Mohanlal Sukhadia University, Udaipur – 313001, India, Tel 9667720689
Received: July 28, 2020 | Published: September 30, 2020
Citation: Meena M, Swapnil P. A review of contagious Coronavirus (SARS-Cov-2) their clinical features, diagnosis, preventions and treatment. J Hum Virol Retrovirolog. 2020;8(4):99-105. DOI: 10.15406/jhvrv.2020.08.00227
Objectives: This review is focused on recent studies of the global threat caused by novel coronavirus. The aim of this study is to understand the origin of the virus, its classification, morphology, genetic structure and mode of infection mechanism with the view towards using this information to develop a cure or for prevention.
Methodology: In Wuhan, China, novel coronavirus pneumonia (SARS-CoV-2) originated and caused a global threat from late December 2019 which afterwards was termed as COVID-19 illness. The genome sequence of this novel coronavirus was found to be very similar with severe acute respiratory syndrome (SARS) and Middle-East respiratory syndrome (MERS) and assigned to betacoronavirus. This novel coronavirus affects the respiratory system of human beings as pneumonia.
Results: Due to this novel coronavirus, WHO declared this a global threat and termed it COVID-19. This coronavirus causes severe health issues in people after direct contact. This disease is more severe for people who are suffering from some previous health issues. To cope with this disease some clinical characterisations are being processed to synthesize significant vaccines and antiviral drugs with the combination of different effective drugs. Therefore, it has been suggested that until a medicine is discovered people have to be careful to prevent this infection from spreading.
Conclusions: Overall, this study is about the pandemic situation of COVID-19. To prepare any vaccine or medicine we have to study the morphology, genetic structure and its infection mechanism. COVID-19 is more dangerous than previous respiratory viruses. Until a medical or scientific team can synthesize a vaccine, we should follow the guidelines given by WHO to limit spread of the coronavirus from person to person.
Keywords: COVID-19; SARS-CoV-2; glycoprotein, clinical diagnosis, prevention
In late December 2019, 27 cases of an unidentified pneumonia were reported in Wuhan city of Hubei Province, China.1–6 The clinical characteristics of this unidentified pneumonia were very similar to viral pneumonia which was caused by novel coronavirus pneumonia (NCP).7 The World Health Organization (WHO) officially named the disease ‘COVID-19’.8 COVID-19 is the seventh discovered coronavirus that also infects humans.9 This novel virus was named as ‘severe acute respiratory syndrome coronavirus 2’ (SARS-CoV-2) by International Committee on Taxonomy of Viruses. This novel coronavirus belongs to the family betacoronavirus, which is a wide class of viruses that are common in nature and have many possible natural, intermediate and final hosts. There are many challenges for the prevention and treatment of this infection. Compared with Middle-East respiratory syndrome coronaviruses (MERS-CoV) the severe acute respiratory syndrome has higher infectivity and transmissibility with low mortality rate.10 After studying genome sequence of SARS-CoV-2, the recognition rates of SARS-CoV-2 genome sequence was found very similar to bat SARS coronavirus (SARSr-CoV-RaTG13).11 This clarified that the origin of SARS-CoV-2 has arisen from bats. On December 12, 2019 the first case of SARS-CoV-2 was reported and further it increased around 79,394 cases with 2,838 deaths,12 then WHO declared this outbreak as global health emergency on January 30, 2020. In the meantime, around 53 countries have been affected by this virus and 6,009 cases have been confirmed with death of 86 patients.13 On February 20, 2020, around 75,761 cases had been confirmed including 2130 deaths across more than 30 countries due to COVID-19.14 Previously, coronaviruses were not reported as pathogenic to humans but in 2002 and 2003 after discovery of severe acute respiratory syndrome (SARS), it has been reported that these coronaviruses mainly CoV-OC43 and CoV-229E were highly pathogenic to humans. Human corona viruses are different types such as alphacoronavirus-229E, alphacoronavirus-NL63, betacoronavirus-OC43, betacoronavirus-HKU1, Middle-East respiratory syndrome (MERS-CoV), severe acute respiratory syndrome (SARS-CoV) and novel coronavirus-COVID-19 (SARS-CoV-2). Mild infections caused by CoV-OC43 and CoV-229E in people with an adequate immune system.15,16
The most affected and exposed Asian countries to coronavirus are China, Mongolia, Cambodia, Laos Philippines, Myanmar and Vietnam through economic and social loss, and mobilization of people.5 Apart from Asian countries, many other countries new cases of coronavirus infections are identified such as in USA and France. The objective of this review article is to provide a preliminary opinion about the disease, the ways of treatment, and prevention in this early stage of the outbreak. This article reviews the opinion about the morphology, genetic structure, way of infection and transmission, pathogenic ability, clinical characterisation, prevention and treatment of SARS-CoV-2 in order to help research, and also provide awareness of this disease to others.
The International Committee on Taxonomy of Viruses (ITCV) classified coronaviruses and put the family Coronaviridae in the order Nidovirales and positioned in subfamily Coronavirinae. According to serological and genomic criteria, Coronavirinae have been divided into four specific genera: Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus.17 In the Betacoronavirus genus 4 discrete lineages (A, B, C, and D) have been positioned. Human coronaviruses such as HCoV-NL63 and HCoV-229E have been assigned to Alphacoronavirus, while HCoV-HKU1 and HCoV-OC43 have been assigned in Betacoronavirus under lineage A, whereas SARS-CoV and MERS-CoV assigned to lineage B and C (Figure 1).
Coronaviruses are non-segmented single-stranded RNA, pleomorphic or spherical virus with a diameter of 80–120 nm.18,19 The surface of the virus is made up of type I trans-membrane proteins such as S (glycoprotein) and HE (hemagglutinin-esterase). The club-like projections and some shorter projections are made up of glycoprotein (S) which is a trimeric type in structure20 and dimeric hemagglutinin-esterase (HE) protein. This type of structure found in betacoronaviruses like HCoV-OC43 and HCoV-HKU1.21 The envelop of the virus is consisted with membrane (M), glycoprotein (S), envelope (E) and transmembrane protein.20,22 The nucleocapsid (N) protein attached to the RNA genome in a beads-on-a-string like manner to form nucleocapsid (Figure 2A).20 The coronavirus genome is a positive-sense, non-segmented, single-stranded RNA, ranging from 27 to 32 kilobases. The genomic RNA contained multiple open reading frames (ORFs) with 5’-capped and 3’-polyadenylated. The gene order of genome comprises replicase-S-E-M-N with numerous accessory proteins encoded ORFs (encoding accessory proteins) in structural genes (Figure 2B). The replicase of coronavirus is encoded by ORF1a and ORF1b which occupied 2/3 of the coronavirus genome and have a tendency to translate directly from genomic RNA. The replication of coronavirus follow several steps such as (1) attachment of coronavirus by recognising receptor on host cell and entry in the host cell through S proteins and result in infection, (2) translation of replicase, (3) transcription, (4) replication, (5) structural proteins translation, and (6) assembly of virion and their release (Figure 3). There are several factors involved in coronavirus replication showed in Table 1. ACE2 (angiotension-converting enzyme 2) used as a receptor by SARS-CoV-2.37 SARS-CoV-2 attached to ACE2 with high affinity (more than 10 fold) than SARS-CoV.38 The mechanism of SARS-CoV-2 infection in humans remains unknown, and more studies are needed to understand the overall mechanism of infection.39
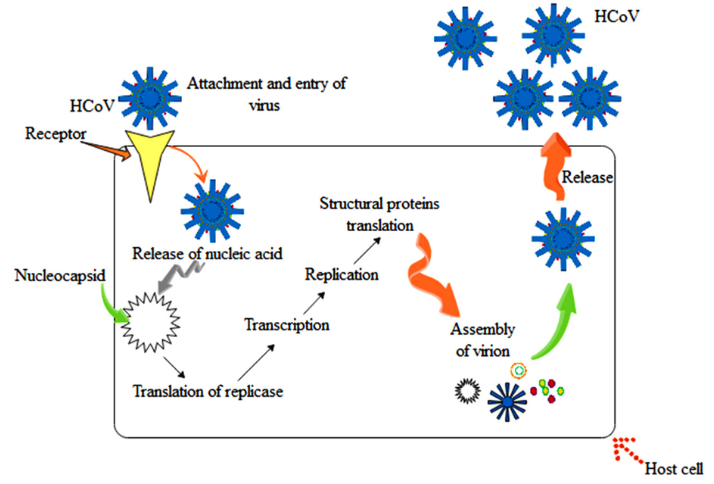
Figure 3 Infection mechanism of coronavirus in humans.
|
S. no. |
Host factors |
HCoV and other CoV |
Reference |
|
1. |
APN |
HCoV-229E |
[23] |
|
2. |
ACE2 |
SARS-CoV |
[24] |
|
3. |
DPP4 (Dipeptidyl peptidase 4) |
MERS-CoV |
[25] |
|
4. |
9-O-acetylated sialic acid |
HCoV-OC43, HCoV-HKU1 |
[26] |
|
5. |
Cathepsin L |
SARS-CoV |
[27] |
|
6. |
Furin |
MERS-CoV |
[28] |
|
7. |
TMPRSS11D |
SARS-CoV, HCoV |
[18] |
|
8. |
VCP |
HCoV-229E, IBV |
[29] |
|
9. |
IFITM |
SARS-CoV, MERS-CoV |
[30] |
|
10. |
IFITM2/IFITM3 |
HCoV-OC43 |
[31] |
|
11. |
GSK3 |
SARS-CoV |
[32] |
|
12. |
hnRNPA1 |
SARS-CoV |
[33] |
|
13. |
PABP |
Bovine-CoV |
[34] |
|
14. |
N-linked Glycosylation enzymes |
SARS-CoV |
[35] |
|
15. |
ER chaperones |
SARS-CoV |
[36] |
Table 1 Different host factors and their coronaviruses (HCoV and others)
The viral infection produced by SARS-CoV-2 in humans has an incubation period of 3 days or 2-14 days.40,41 However in Hubei Province the incubation period of SARS-CoV-2 is recorded for 27 days on February, 22.37 The symptoms of COVID-19 disease are fever, cough and fatigue, diarrhoea and vomiting.40,42 The severe cases of COVID-19 include acute heart injury, acute respiratory distress syndrome, secondary infection11 and arrhythmia, abnormal liver function and impaired renal function.7,43,44 In a report it has been studied that 214 patients with COVID-19 showed neurological manifestations45 and liver disease microvesicular steatosis.46 An important tool like chest computed tomography (CT) scan is used to diagnose for pneumonia47 and these scans could diagnose 97% of COVID-19 patients.48 Next-generation sequencing and real-time reverse transcription polymerase chain reaction (RT-PCR) were also used for the confirming COVID-19 positive nucleic acid from throat swabs, sputum and secretions of the respiratory samples.49
Successful diagnosis of COVID-19 may involve long testing times and there is some potential for false negative results. The standard diagnostic of COVID-19 require nucleic acid of SARS-CoV-2 virus. Notably, the diagnosis of nucleic acid of SARS-CoV-2 showed low sensitivity and high specificity. In Hubei Province the detection of COVID-19 treatment based on the process related to pneumonia diagnosis and their treatment.50 The diagnosis and treatment of coronavirus related to pneumonia has been eliminated due to increased number of COVID-19 infected patients from outside of Hubei Province.51 Furthermore, using SHERLOCK technology rapid test (1 h) of SARS- CoV-2 has been developed but the clinical verifications have not been done in this technology. Once the technology proved by the researchers, it may be favourable for the rapid detection of the COVID-19. A group of researchers at Peking University reported rapid test for the SARS-CoV-2 through rapid transcriptome sequencing construction using sequencing SHERRY library.52,53
WHO and US Centers for Disease Control and Prevention bodies issued various advices for the control and prevention of the COVID-19.1,3,5 According to these bodies travel should be avoided to high risk infected areas, contact with symptomatic individuals, and consuming meat from high-risk COVID-19 outbreak regions. They should promote to hygiene measurements like use of hand wash (every 15–20 min), PPE kit, face-mask and covering the face with elbow during sneezing. They also recommended the isolation at home of COVID-19 positive patients or suspected patients. At home, sunlight and ventilation should be encouraged for the destruction of virus. Healthcare workers are more prone to this disease because they may come in direct contact during treatment of COVID-19 patients. According to Chang et al.,54 in 2002 around 21% health workers were affected during SARS outbreak. The doctors have confirmed that these viruses can cause death too, so it is very important to protect healthcare workers to prevent transmission of pathogenic infection to other healthy people. Currently, there is no widespread clinical treatment for COVID-19. However, a common treatment approach is to minimize virus transmission in non-hospitalized patients by maintaining hydration and nutrition to control cough and fever symptoms. Antibiotics and antiviral drugs users should avoid oseltamivir drugs in confirmed cases of SARS-CoV-2. In case of hypoxic patients they can use high flow nasal cannula (HFNC), oxygen through nasal prongs, ventilator and face mask, and in some cases renal replacement therapy is required. In China, low dose of corticosteroids are recommended for the treatment of COVID-19.55,56 Some antiviral drugs like lopinavir-ritonavir and ribavirin have been recommended in SARS and MERS. It has been found that the SARS patients treated with a combination of lopinavir-ritonavir and ribavirin showed better results compared to ribavirin alone.11 Severe immune reactions in patients can be treated with the use of glucocorticoids.57 In the case of children, the use of methylprednisolone should be given around 1–2 mg/kg/day for 5 days maximally.58,59 For the recommendation of these drugs more evidence is required. Some other clinical methods such as use of arbidol (antiviral drug of Russia and China), interferons, chloroquine, intravenous immunoglobulin and plasma of recovered patients from COVID-19 have been used for the treatment of COVID-19.60–62 As well, some Chinese herbs have been recommended in Chinese guidelines as a traditional method.61 Unfortunately, at present, there is no effective vaccine or antiviral treatment available for COVID-19, so currently random multicentre controlled clinical trial are being monitored to measure the efficiency and protection of COVID-19 patients.63
This study reviews current aspects of the disease caused by coronavirus. Most of the early studies that appeared and were published about novel coronavirus were about the epidemiology, clinical characterization, diagnosis and about prevention and control. This review discusses that how to minimize the spread of COVID-19 by looking at clinical interventions. This novel coronavirus outbreak remains a challenge for the economic, public health infrastructure and medical condition of more than 200 countries. WHO reported that globally, on September 17, 2020, approx. 30,087,248 cases of COVID-19 have been confirmed including 945,978 deaths. In India, 83,230 deaths and 5,118,253 active cases have been reported till date and the impact of coronavirus is increasing gradually day by day. Therefore, significant effort is required to plan inclusive measures to avoid future outbreaks of zoonotic origin.
None.
The authors declare no conflicts of interest.

©2020 Meena, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.