Journal of
eISSN: 2373-6453


Editorial Volume 11 Issue 1
1Microbiology Postgraduate Program, School of Medical Sciences, Rio de Janeiro State University, Brazil
2Department of Diagnosis and Therapeutics, School of Dentistry, Rio de Janeiro State University, Brazil
3School of Medicine and Surgery, Federal University of the State of Rio de Janeiro, Brazil
Correspondence: Guilherme Goulart Cabral-Oliveira, Rio de Janeiro State University, Biomedical Center, School of Medical Sciences, Boulevard Vinte e Oito de Setembro - 87 Fundos - Terceiro andar - Laboratório 3, Vila Isabel 20551030 - Rio de Janeiro, RJ, Brazil, Tel +55 2128688280
Received: January 07, 2024 | Published: January 8, 2024
Citation: Cabral-Oliveira GG, Motta ICM, Silva-Junior GO. A discussion about HPV vaccine and oropharyngeal cancer, in Brazil. J Hum Virol Retrovirol. 2024;11(1):1-2. DOI: 10.15406/jhvrv.2024.11.00272
Vaccines are an effective method to prevent and control infectious diseases. Brazil, through its public health system, has one of the most complete and free immunization programs, being referred to worldwide.1,2 In 2014, the program included the Human Papillomavirus (HPV) quadrivalent recombinant vaccine, which presents the L1-protein of virus-types 6, 11, 16, and 18, more associated with oncogenic tumors.3 HPV-driven cancer, like cervical cancer, promotes a high socio-economic impact and presents public health relevance and the vaccine is an effective method for preventing them.4 In the head and neck region, the virus-type 16 is associated with developing oropharyngeal cancer.5–8 Oropharyngeal cancer shows a high score of morbimortality, principally due to your localization, the oropharyngeal region is not explored and visualized contributing to a late diagnosis, furthermore, their nearness noble structures like the thyroid gland and the brain promote a difficult treatment with chemo and radiotherapy.5,7–9
Despite the relevance of the HPV vaccine, the total of doses of the vaccine applied has decreased from 2014 to 2022, in Brazil (Figure 1). Comparing the men's and women's doses of the vaccine applied, in the three first years (2014, 2015, and 2016) there was a great discrepancy in the score, however, these differences decreased from 2017 to 2022 (Figure 1). In Brazil, adherence to the HPV vaccine does not reach the level recommended by the World Health Organization, of 90% for girls between 9 and 14 years old. According to a study by the Cancer Foundation, with data from 2013 to 2020, 76% of the target audience took the first dose and only 56% took the two doses provided for in the Brazilian vaccination schedule. Concerning boys, the numbers are also worrying: vaccination coverage fell from 61.55% in 2019 to 52.16% in 2022.10 It is important to highlight, however, that the HPV quadrivalent vaccine has proven efficient for reducing the burden of oral HPV infection11 which may impact the burden of HPV-driven head and neck cancer, and thus efforts should be made to increase vaccine coverage.10
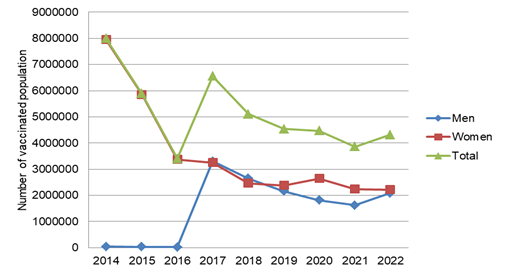
Figure 1 Distribution of the number of doses of the Human Papillomavirus (HPV) vaccine, administered by sex and according to the dose (first or second) of the vaccination schedule, in Brazil, 2014- 2022. Data was obtained freely from the Brazilian Public Health System (DataSUS, http://tabnet.datasus.gov.br, Dec 2023).
In conclusion, the HPV vaccine could be an effective prevention method for papillomavirus disease-associated, principally oncogenic issues. Recently, Brazil made available the HPV nonavalent recombinant vaccine that offers immunity to nine virus-types (6, 11, 16, 18, 31, 33, 45, 52, and 58) increasing the prevention and development of oncogenic tumors. The presence of HPV has been considered the predisposing factor for oropharyngeal cancer. However, have few papers associating the HPV vaccine and oropharyngeal cancer incidences in this country, which need future research.
None.
None.
Author declares that there is no conflict of interest.

©2024 Cabral-Oliveira, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.